01 Nov Puccinia
Puccinia
Background
Puccinia is the type genus of the family Pucciniaceae in the order of rust fungi, Pucciniales (Basidiomycota). Puccinia has approximately 4,000 named species (Kirk et al. 2008) and is a widespread genus of plant pathogens that have shaped history. For example, Puccinia graminis, the type species of Puccinia, was investigated as a biological warfare agent in the cold war (Line and Griffith 2001). It was the impetus for breeding wheat cultivars resistant to a disease that started the Green Revolution, lead by 1970 Nobel Laureate, Norman Borlaug (Zeyen et al. 2014). Epidemics of stem rust of wheat caused by P. graminis remain a threat with the emergence of races such as Ug99 (Singh et al. 2011). Other species of Puccinia are also serious pathogens of grasses (Poaceae), including P. coronata and P. striiformis (Kirk et al. 2008). Rusts of Asteraceae, e.g., P. helianthi, and rusts of Fabaceae in the closely related genus Uromyces, e.g., U. viciae-fabae, U. appendiculatus and U. ciceris-arietini, are important pathogens of cultivated fodder and food crops.
Among the ca. 120 to 160 genera of rust fungi (Cummins and Hiratsuka 2003; Kirk et al. 2008), Puccinia is readily recognized by the two-celled teliospores and the shape of the spermogonia (Cummins and Hiratsuka 2003). Uromyces with one-celled teliospores is typically differentiated from Puccinia, although some species of Puccinia have both one-celled (mesospores) and two-celled teliospores, e.g., P. lagenophorae. Teliospore morphology is homoplasious, and Puccinia and Uromyces were polyphyletic in systematic studies based on the LSU and SSU regions of nuclear ribosomal DNA (Maier et al. 2007; Aime 2006), and the two nuclear genes: elongation factor and β- tubulin (Van der Merwe et al. 2007). Some rust fungi have teliospores morphologically similar to Puccinia, but are not closely related or have an uncertain systematic position. For example, Allodus podophylli has two-celled teliospores convergent with Puccinia. A systematic analysis based on the nLSU and nSSU regions of rDNA determined Allodus and Puccinia were unrelated (Minnis et al. 2012). Puccinia psidii, which spread from South America to much of the Pacific region and South Africa, now infects 30 genera of Myrtaceae out of its natural host range (Pegg et al. 2013). It has two-celled teliospores, but its placement within the Pucciniales is unknown. Phylogenetic analyses of the nLSU and nSSU (Pegg et al. 2013) and the protein-coding gene beta-tubulin (Van Der Merwe et al. 2008) indicated that P. psidii was sister to the Pucciniaceae. Several families and genera of rust fungi are polyphyletic, namely the Raveneliaceae, Phakopsoraceae, and Pucciniaceae. These polyphyletic families and genera await resolution by molecular phylogenetic analyses.
Species identification and numbers
Rust fungi are usually considered host-specific (Cummins and Hiratsuka 2003), although some, e.g., Puccinia psidii and P. lagenophorae, infect multiple host genera (McTaggart et al. 2014; Pegg et al. 2013). Some species of rust fungi are heteroecious, requiring two hosts in different families to complete their life cycle, e.g., P. graminis on Triticum (Poaceae) and Berberis (Berberidaceae).
Rust fungi have a complicated life cycle with up to five spore states (Cummins and Hiratsuka 2003). Consequently, up to three names have been proposed for the same taxon based on different life cycle stages. To add to the confusion, there are two systems of terminology that describe these spore states, one based on morphology (Laundon 1967), and the other on ontogeny (Arthur and Kern 1926; Cummins and Hiratsuka 2003; Hiratsuka 1973). These systems of terminology were summarised by Hennen and Hennen (2000).
Species of rust fungi are often identified on the basis of their host specificity, and monographs were organized by plant family (Sydow and Sydow 1904; McAlpine 1906; Cummins 1971, 1978). Morphological characters of the teliospores and urediniospores, such as size, apex shape, and wall thickness, ornamentation, and germ pore position and number, are useful for species identification.
Molecular diagnostic tools have been developed for some species of Puccinia based on the ITS region of rDNA, e.g., P. coronata (Beirn et al. 2011; Pfunder et al. 2001), P. kuehnii (Glynn et al. 2010) and P. psidii (Langrell et al. 2008). The ITS region has successfully distinguished phylogenetic species in Uromyces (Barilli et al. 2011) and it was used in combination with TEF to resolve the taxonomy of P. melampodii (Seier et al. 2009). However, the ITS region was polymorphic in Puccinia lagenophorae (Littlefield et al. 2005; Scholler et al. 2011), and Morin et al. (2009) discovered a paralogous copy of the ITS region, which may have resulted from a hybridization event. A paralogous copy of the ITS region was also reported in P. kuehnii in the study by Virtudazo et al. (2001). Polymorphisms and paralogous copies are caveats for studies based on the ITS region in rust fungi.
Molecular phylogeny
Large-scale systematic studies of rust fungi have focused mainly on the SSU and LSU regions of rDNA (Aime 2006; Beenken et al. 2012; Dixon et al. 2010; Maier et al. 2003, 2007; Minnis et al. 2012; Wingfield et al. 2004; Yun et al. 2011) (Table). Protein coding genes such as beta-tubulin (Morin et al. 2009; Van der Merwe et al. 2007, 2008) and elongation factor (TEF) (Seier et al. 2009; Van der Merwe et al. 2007) were successfully used at the family, genus and species level in rust fungi, although beta-tubulin required cloning rather than direct sequencing of PCR product. Liu et al. (2013) included ITS, beta-tubulin, ribosomal polymerase subunit 2 (RPB2) and cytochrome c oxidase subunit 1 (COI) in a systematic study to resolve the P. coronata species complex. They discussed the difficulty of PCR amplification of older herbarium specimens, and that DNA repair was successful in some cases. Vialle et al. (2009) compared mitochondrial genes to rDNA markers in two genera of rusts, Chrysomyxa and Melampsora. They found rDNA had better species resolution than mitochondrial genes. Mitochondrial genes were since used in studies of the genera Chrysomyxa (Feau et al. 2011) and Dasyspora (Beenken et al. 2012), but have not yet been used for Puccinia.
Table Puccinia. Details of the isolates used in the phylogenetic tree
| Species | Isolate | Host | GenBank accession no. | |
| LSU | SSU | |||
| Aecidium kalanchoe | BPI 843633 | Kalanchoe blossfeldiana | AY463163 | DQ354524 |
| Allodus podophylli | BPI 842277 | Podophyllum peltatum | DQ354543 | DQ354544 |
| Caeoma torreyae | ECS 553 | Torreya californica | AF522183 | AY123284 |
| Cumminsiella mirabilissima | BPI 871101 | Mahonia aquifolium | DQ354531 | DQ354530 |
| Helicobasidium purpureum | CBS324.47 | Not provided | AY885168 | D85648 |
| Dietelia portoricensis | BPI 844288 | Mikania micrantha | DQ354516 | AY125414 |
| Miyagia pseudosphaeria | BPI 842230 | Sonchus oleraceus | DQ354517 | AY125411 |
| Pileolaria toxicodendri | BPI 871761 | Toxicodendron sp. | DQ323924 | AY123314 |
| Prospodium lippiae | BPI 843901 | Aloysia plystachya | DQ354555 | DQ831024 |
| P. tuberculatum | BRIP 57630 | Lantana camara | KJ396195 | KJ396196 |
| Puccinia caricis | BPI 871515 | Grossularia sp. | DQ354514 | DQ354515 |
| P. convolvuli | BPI 871465 | Calystegia sepium | DQ354512 | DQ354511 |
| P. coronata | Rhamnus cathartica | DQ354526 | DQ354525 | |
| P. dampierae | BRIP 57724 | Dampiera linearis | KF690688 | NA |
| P. gilgiana | BRIP 57719 | Lechenaultia linarioides | KF690691 | NA |
| P. graminis | NA | Not provided | AF5221779 | NA |
| P. haemodori | BRIP 56965 | Anigozanthus sp | KF690692 | NA |
| P. hemerocallidis | BPI 843967 | Hemerocallis sp. | DQ354519 | DQ354518 |
| P. hordei | BPI 871109 | Poaceae | DQ354527 | DQ415278 |
| P. lagenophorae | BRIP 57563 | Emilia sonchifolia | KF690696 | NA |
| P. menthae | BPI 871110 | Cunila origanoides | DQ354513 | AY123315 |
| P. psidii | BRIP 57991 | Melaleuca leucadendra | KF318443 | KF318455 |
| P. poarum | NA | Tussilago sp. | DQ831028 | DQ831029 |
| P. polysora | BPI 863756 | Zea mays | GU058024 | NA |
| P. saccardoi | BRIP 57725 | Scaevola spinescens | KF690701 | NA |
| P. smilacis | BPI 871784 | Smilax rotundifolia | DQ354533 | DQ354532 |
| P. stylidii | BRIP 60107 | Stylidium armeria | KJ622214 | NA |
| P. ursiniae | BRIP 57993 | Ursinia anthemoides | KF690705 | NA |
| P. violae | BPI 842321 | Viola cucullata | DQ354509 | DQ354508 |
| P. xanthosiae | BRIP 57729 | Xanthosia rotundifolia | KF690706 | NA |
| Pucciniosira solani | NA | Solanum aphyodendron | EU851137 | NA |
| Uromyces appendiculatus | NA | Phaseolus vulgaris | AY745704 | DQ354510 |
| U. ari-triphylli | BPI 871111 | Arisaena triphyllum | DQ354529 | DQ354528 |
| U. scaevolae | BRIP 60113 | Selliera radicans | KJ622213 | NA |
| U. viciae-fabae | NA | Pisum sp. | AY745695 | NA |
Ex-type (ex-epitype) strains are bolded and marked with an * and voucher stains are bolded
Recommended genetic markers
- The large subunit of nrDNA (LSU)–is useful for genus and species-level identification of all rust fungi
- The internal transcribed spacer (ITS)–is useful for species-level identification, but may contain polymorphic sites and paralogous copies. Rust specific primers are recommended.
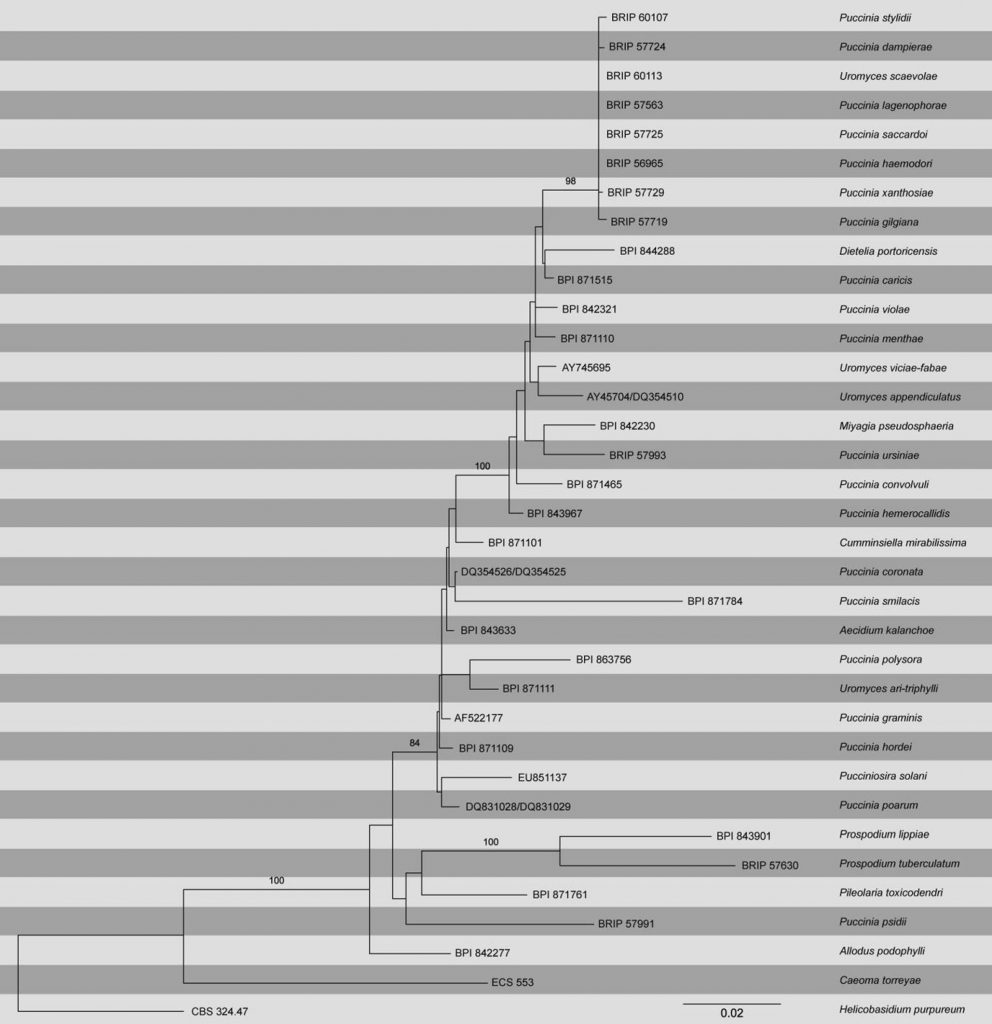
Rusts are obligate biotrophs and difficult to maintain in pure culture, which has posed a challenge for DNA extraction (Aime 2006). This is reflected by the relatively few species of Puccinia represented in GenBank, for example, there are ~110 species of Puccinia represented by the ITS and LSU regions of rDNA. This is less than 3 % of the estimated 4,000 species of Puccinia (Kirk et al. 2008). Reliance on molecular identification for some species of Puccinia is not recommended. For example, McTaggart et al. (2014) determined that several species of Puccinia on different plant families in Australia had near-identical ITS and LSU rDNA sequences (Fig. Puccinia).
Fig. Puccinia. Phylogram obtained from an ML search in RAxML with the SSU and LSU regions of nrDNA. Bootstrap values (≥70 %) from an ML search with 1,000 replicates above nodes; posterior probabilities (≥0.95) from Bayesian inference below nodes. Puccinia and Uromyces are polyphyletic, and genera such as Cumminsiella, Dieteila, Miyagia and Pucciniosira are paraphyletic. The LSU region is not sufficient to distinguish closely related taxa in Australia as seen in the P. lagenophorae clade

No Comments