17 Sep Alternaria
Alternaria Nees, Syst. Pilze (Würzburg): 72 (1816) [1816–17]
For synonyms see Index Fungorum (2018)
Background
Alternaria was established by Nees von Esenbeck (1816) and species of Alternaria are known as serious plant pathogens (Nishimura et al. 1978; Peever et al. 2002; Thomma 2003; Lawrence et al. 2013; Woudenberg et al. 2013, 2015) and saprobes (Wanasinghe et al. 2018). Alternaria species have been also recorded as endophytes in grasses, angiosperms, rice and other herbaceous plants and shrubs (Fisher and Petrini 1992; Schulz et al. 1993; Rosa et al. 2009; Polizzotto et al. 2012) and they have been also isolated from soil (Hong and Pryor 2004). Many Alternaria species are saprobic on a variety of plant tissues in different habitats (Thomma 2003; Liu et al. 2015; Wanasinghe et al. 2018). Some Alternaria species, such as A. alternata, produce host-specific toxins (Hyde et al. 2018a). Several taxa are also important postharvest pathogens, e.g. A. alternata and A. solani (El-Goorani and Sommer 1981; Reddy et al. 2000), or airborne allergens causing upper respiratory tract infections and asthma in humans (Mitakakis et al. 2001; Woudenberg et al. 2015; Hyde et al. 2018a). Mycotoxins produced by Alternaria have been found in many crops including grapevine, olive, orange and tomato (Logrieco et al. 2003). This genus has been considered as one of the most important phytopathogens, especially in temperate regions (Ariyawansa et al. 2015; Wanasinghe et al. 2018).
Classification – Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae
Type species – Alternaria alternata (Fr.) Keissl., Beih. bot. Zbl., Abt. 2 29: 434 (1912)
Distribution – Worldwide
Disease symptoms – Leaf blotch, leaf spot, stem canker and stem end rots
Alternaria generally infects the aerial parts of its host. On leafy vegetables the infection typically starts as a small, circular, dark spot. As the disease progresses, the circular spots may enlarge and are usually gray, gray-tan or near black in colour. In some cases the spots may develop in a target pattern of concentric rings and if the host leaves are large enough unrestricted symptom development can be observed. The lesion may often be covered with a fine, black, fuzzy growth (Agrios 1997). On roots, tubers, stems and fruits dark brown to black sunken lesions with concentric rings may occur. Lesions enlarge and may girdle the stem, eventually killing the plant. Fruits that are harvested from infested plants have brown or black necrotic sunken lesions (Agrios 1997; Wenneker et al. 2018). The above symptoms can be observed when the infection is caused by A. alternata, A. arborenses, A. tenuissima (Diskin et al. 2017). Alternaria brassicola produces black sooty coloured spores within the leaf spot (Kreis et al. 2016). Purple blotch disease of Allium sp. is caused by A. porri, which initially appears as small whitish necrotic lesions on leaves, becoming large, sunken and subsequently turning brown and dark (Hahuly et al. 2018).
Hosts – Has a wide range of hosts including the families Amarylidaceae, Apiaceae, Brassicaceae, Fabaceae, Lamiaceae, Rosaceae, Rutaceae, Solanaceae, Vitaceae plus many ornamental plants and a number of weeds (Farr and Rosmann 2018).
Morphological based identification and diversity
The asexual morphs of Alternaria are ubiquitous in different environments and characterized by distinct, single, simple or irregular, loosely branched, solitary conidiophores, which may be in fascicles, and by the production of dark coloured phaeodictyospores in chains, the conidia often having a beak of tapering apical cells (Woudenberg et al. 2013). Sexual morphs have small, globose to ovoid, dark brown, papillate ostiolate ascomata, mostly 8-spored, bitunicate asci with a pedicel and ocular chamber, and muriform ascospores (e.g. section Crivellia, Eureka, Infectoria; Woudenberg et al. 2013; Ariyawansa et al. 2015a; Wanasinghe et al. 2018). Neergaard (1945) divided Alternaria into three major sections, Brevicatenatae, Longicatenatae and Noncatenatae, based on conidial catenation. However, this division is unreliable as catenation is affected by growth conditions. Simmons (1992, 1995) arranged several species groups within Alternaria based on the morphological similarity among species,. Some other genera, such as Stemphylium (Wallroth 1833) and Ulocladium (Preuss 1851) also produce phaeodictyosporic conidia and are morphologically similar to Alternaria, and this has further led to taxonomic complications. Simmons (2007) revised Alternaria taxonomy based on morphology and 275 species were recognized. At the same time, Simmons (2007) proposed three new genera Alternariaster, Chalastospora and Teretispora, for some species that were previously described in Alternaria. The Alternaria complex currently comprises 24 sections and six monophyletic lineages (Woudenberg et al. 2013).
Colony and conidial morphology are the primary characters to identify species within this genus (Ellis 1971, 1976; Simmons 1992). Conidia in some sections are mostly dictyosporous, e.g. Alternata and Japonicae, while some are mostly phragmosporous, e.g. Alternantherae and Nimbya. Species in some sections have long apical narrow beaks or secondary conidiophores, e.g. Alternantherae, Dianthicola and Porri, while such characters are absent in other sections, e.g. Chalastospora, Gypsophilae and Ulocladium. However, in some sections overlapping conidial morphology is observed, which makes identification of Alternaria based on morphology challenging. For example, dictyospores and phragmospores can be found in the same section, such as Infectoriae and Phragmosporae. Therefore, the use of DNA sequence data is very important in resolving Alternaria taxonomy.
Molecular based identification and diversity
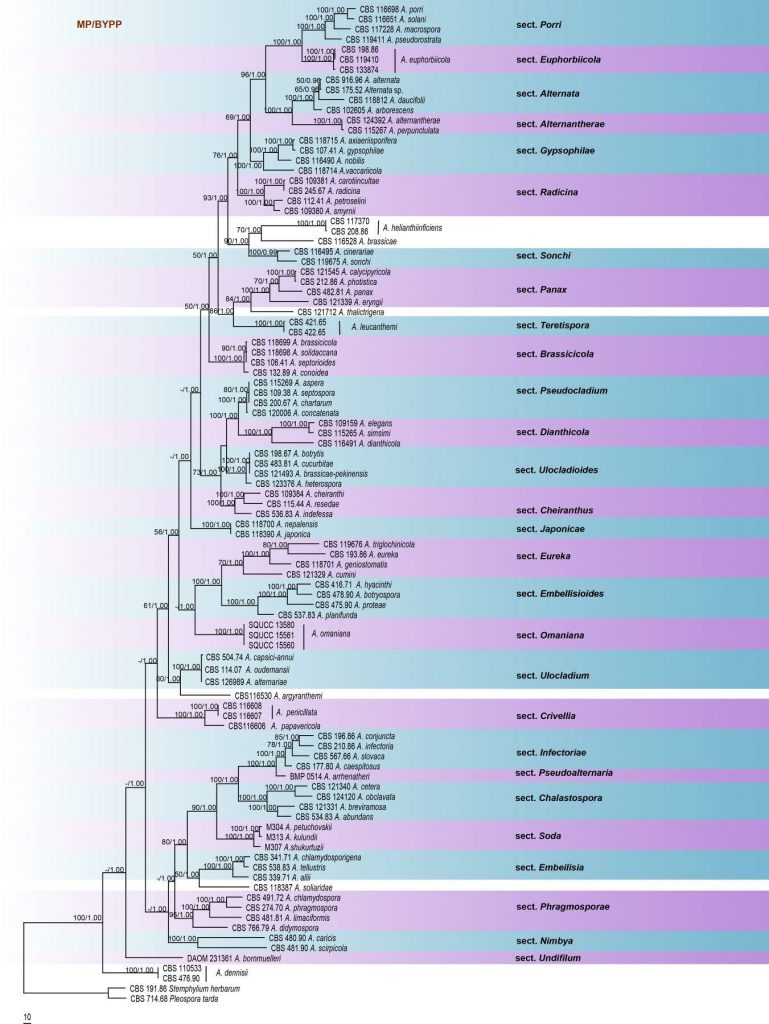
Molecular phylogeny has revealed multiple polyphyletic taxa within Alternaria and Alternaria species clades, which do not always correlate to morphological species-groups (Inderbitzin et al. 2006; Runa et al. 2009; Lawrence et al. 2012). Pryor and Gilbertson (2000) elucidated relationships among Alternaria, Stemphylium and Ulocladium based on ITS and SSU sequence data and revealed that Stemphylium species were phylogenetically distinct from Alternaria and Ulocladium species. Most Alternaria and Ulocladium clustered together in a large Alternaria/Ulocladium clade (Pryor and Gilbertson 2000). Chou and Wu (2002) confirmed that filament-beaked Alternaria species constitute a monophyletic group distinct from the other members in this genus and hypothesized that this group is evolutionary distinct based on ITS sequence based phylogenies. Two new species groups, A. panax and A. gypsophilae were introduced by Lawrence et al. (2013) with phylogenetic evidence, and they accepted eight well supported asexual species-sections within Alternaria, while the taxa with known sexual morphs, the A. infectoria species-groups, were not given similar rank. Woudenberg et al. (2013) delineated taxa within Alternaria and allied genera based on SSU, LSU, ITS, GAPDH, RPB2 and TEF1- α sequence data. The generic circumscription of Alternaria was emended and 24 internal clades in the Alternaria complex were treated as sections, together with six monotypic lineages. Ariyawansa et al. (2015a) revised the classification of Pleosporaceae with a major focus on Alternaria and allied genera. Agreeing with Woudenberg et al. (2013), six monotypic lineages and 24 internal clades were recognized, with Xenobotryosphaeria clustering within A. infectoria. The present study reconstructs the phylogeny of Alternaria based on analyses of a combined SSU, LSU, RPB2, ITS, GAPDH and TEF1-α sequence data. The phylogenetic tree is updated with recently introduced Alternaria species, and the resulting tree corresponds to previous studies (Woudenberg et al. 2013; Ariyawansa et al. 2015a; Thambugala et al. 2017).
Recommended genetic markers (Genus level) – LSU and SSU
Recommended genetic markers (Species level) – ITS, GAPDH, RPB2 and TEF1-α
GAPDH is the common species marker used in identification of Alternaria species. Combined GAPDH with ITS, RPB2 and TEF1- α provides satisfactory resolution for resolving species.
Accepted number of species: There are 730 species epithets in Index Fungorum (2018) under this genus. However, only 87 have DNA sequence data.
References: Simmons 2007 (morphology), Ariyawansa et al. 2015a, Lawrence et al. 2013, Woudenberg et al. 2013, 2015 (morphology, phylogeny).
Table Details of the Alternaria isolates used in the phylogenetic analyses.
| Species name | Strain number | GenBank accession numbers | |||||
| SSU | LSU | RPB2 | ITS | GPDH | tef1 | ||
| Alternaria abundans | CBS 534.83 | KC584581 | KC584323 | KC584448 | JN383485 | KC584154 | KC584707 |
| A. alternantherae | CBS 124392 | KC584506 | KC584251 | KC584374 | KC584179 | KC584096 | KC584633 |
| A. alternariae | CBS 126989 | KC584604 | KC584346 | KC584470 | AF229485 | AY278815 | KC584730 |
| A. alternate | CBS 916.96 | KC584507 | DQ678082 | KC584375 | AF347031 | AY278808 | KC584634 |
| A. arborescens | CBS 102605 | KC584509 | KC584253 | KC584377 | AF347033 | AY278810 | KC584636 |
| A. argyranthemi | CBS 116530 | KC584510 | KC584254 | KC584378 | KC584181 | KC584098 | KC584637 |
| A. arrhenatheri | BMP 0514 | – | – | – | JQ693680 | JQ693629 | – |
| A. aspera | CBS 115269 | KC584607 | KC584349 | KC584474 | KC584242 | KC584166 | KC584734 |
| A. atra | CBS 195.67 | KC584608 | KC584350 | KC584475 | AF229486 | KC584167 | KC584735 |
| A. axiaeriisporifera | CBS 118715 | KC584513 | KC584257 | KC584381 | KC584184 | KC584101 | KC584640 |
| A. bornmueller | DAOM 231361 | KC584624 | KC584366 | KC584491 | FJ357317 | FJ357305 | KC584751 |
| A. botryospora | CBS 478.90 | KC584594 | KC584336 | KC584461 | AY278844 | AY278831 | KC584720 |
| A. botrytis | CBS 197.67 | KC584609 | KC584351 | KC584476 | KC584243 | KC584168 | KC584736 |
| A. brassicae | CBS 116528 | KC584514 | KC584258 | KC584382 | KC584185 | KC584102 | KC584641 |
| A. brassicae- pekinensis | CBS 121493 | KC584611 | KC584353 | KC584478 | KC584244 | KC584170 | KC584738 |
| A. brassicicola | CBS 118699 | KC584515 | KC584259 | KC584383 | JX499031 | KC584103 | KC584642 |
| A. breviramosa | CBS 121331 | KC584574 | KC584318 | KC584442 | FJ839608 | KC584148 | KC584700 |
| A. calycipyricola | CBS 121545 | KC584516 | KC584260 | KC584384 | KC584186 | KC584104 | KC584643 |
| A. capsici-annui | CBS 504.74 | KC584517 | KC584261 | KC584385 | KC584187 | KC584105 | KC584644 |
| A. caricis | CBS 480.90 | KC584600 | KC584342 | KC584467 | AY278839 | AY278826 | KC584726 |
| A. carotiincultae | CBS 109381 | KC584518 | KC584262 | KC584386 | KC584188 | KC584106 | KC584645 |
| A. cetera | CBS 121340 | KC584573 | KC584317 | KC584441 | JN383482 | AY562398 | KC584699 |
| A. chartarum | CBS 200.67 | KC584614 | KC584356 | KC584481 | AF229488 | KC584172 | KC584741 |
| A. cheiranthi | CBS 109384 | KC584519 | KC584263 | KC584387 | AF229457 | KC584107 | KC584646 |
| A. chlamydospora | CBS 491.72 | KC584520 | KC584264 | KC584388 | KC584189 | KC584108 | KC584647 |
| A. chlamydosporigena | CBS 341.71 | KC584584 | KC584326 | KC584451 | KC584231 | KC584156 | KC584710 |
| A. cinerariae | CBS 116495 | KC584521 | KC584265 | KC584389 | KC584190 | KC584109 | KC584648 |
| A. concatenate | CBS 120006 | KC584613 | KC584355 | KC584480 | KC584246 | AY762950 | KC584740 |
| A. conjuncta | CBS 196.86 | KC584522 | KC584266 | KC584390 | FJ266475 | AY562401 | KC584649 |
| A. conoidea | CBS 132.89 | KC584585 | KC584327 | KC584452 | AF348226 | FJ348227 | KC584711 |
| A. consortialis | CBS 104.31 | KC584615 | KC584357 | KC584482 | KC584247 | KC584173 | KC584742 |
| A. cucurbitae | CBS 483.81 | KC584616 | KC584358 | KC584483 | FJ266483 | AY562418 | KC584743 |
| A. cumini | CBS 121329 | KC584523 | KC584267 | KC584391 | KC584191 | KC584110 | KC584650 |
| A. daucifolii | CBS 118812 | KC584525 | KC584269 | KC584393 | KC584193 | KC584112 | KC584652 |
| A. dennisii | CBS 110533 | KC584586 | KC584328 | KC584453 | KC584232 | KC584157 | KC584712 |
| A. dennisii | CBS 476.90 | KC584587 | KC584329 | KC584454 | JN383488 | JN383469 | KC584713 |
| A. dianthicola | CBS 116491 | KC584526 | KC584270 | KC584394 | KC584194 | KC584113 | KC584653 |
| A. didymospora | CBS 766.79 | KC584588 | KC584330 | KC584455 | FJ357312 | FJ357300 | KC584714 |
| A. elegans | CBS 109159 | KC584527 | KC584271 | KC584395 | KC584195 | KC584114 | KC584654 |
| A. embellisia | CBS 339.71 | KC584582 | KC584324 | KC584449 | KC584230 | KC584155 | KC584708 |
| A. eryngii | CBS 121339 | KC584529 | KC584273 | KC584397 | JQ693661 | AY562416 | KC584656 |
| A. eureka | CBS 193.86 | KC584589 | KC584331 | KC584456 | JN383490 | JN383471 | KC584715 |
| A. geniostomatis | CBS 118701 | KC584532 | KC584276 | KC584400 | KC584198 | KC584117 | KC584659 |
| A. gypsophilae | CBS 107.41 | KC584533 | KC584277 | KC584401 | KC584199 | KC584118 | KC584660 |
| A. helianthiinficiens | CBS 117370 | KC584534 | KC584278 | KC584402 | KC584200 | KC584119 | KC584661 |
| A. helianthiinficiens | CBS 208.86 | KC584535 | KC584279 | KC584403 | JX101649 | KC584120 | EU130548 |
| A. heterospora | CBS 123376 | KC584621 | KC584363 | KC584488 | KC584248 | KC584176 | KC584748 |
| A. hyacinthi | CBS 416.71 | KC584590 | KC584332 | KC584457 | KC584233 | KC584158 | KC584716 |
| A. indefessa | CBS 536.83 | KC584591 | KC584333 | KC584458 | KC584234 | KC584159 | KC584717 |
| A. infectoria | CBS 210.86 | KC584536 | KC584280 | KC584404 | DQ323697 | AY278793 | KC584662 |
| A. japonica | CBS 118390 | KC584537 | KC584281 | KC584405 | KC584201 | KC584121 | KC584663 |
| A. kulundii | M313 | KJ443087 | KJ443132 | KJ443176 | KJ443262 | KJ649618 | – |
| A. leucanthemi | CBS 421.65 | KC584605 | KC584347 | KC584472 | KC584240 | KC584164 | KC584732 |
| A. leucanthemi | CBS 422.65 | KC584606 | KC584348 | KC584473 | KC584241 | KC584165 | KC584733 |
| A. limaciformis | CBS 481.81 | KC584539 | KC584283 | KC584407 | KC584203 | KC584123 | KC584665 |
| A. macrospora | CBS 117228 | KC584542 | KC584286 | KC584410 | KC584204 | KC584124 | KC584668 |
| A. nepalensis | CBS 118700 | KC584546 | KC584290 | KC584414 | KC584207 | KC584126 | KC584672 |
| A. nobilis | CBS 116490 | KC584547 | KC584291 | KC584415 | KC584208 | KC584127 | KC584673 |
| A. obclavata | CBS 124120 | KC584575 | FJ839651 | KC584443 | KC584225 | KC584149 | KC584701 |
| A. oudemansii | CBS 114.07 | KC584619 | KC584361 | KC584486 | FJ266488 | KC584175 | KC584746 |
| A. omaniana | SQUCC 13580 | MK878559 | MK878556 | MK880893 | MK878562 | MK880899 | MK880896 |
| A. omaniana | SQUCC 15560 | MK878560 | MK878557 | MK880894 | MK878563 | MK880900 | MK880897 |
| A. omaniana | SQUCC 15561 | MK878561 | MK878558 | MK880895 | MK878564 | MK880901 | MK880898 |
| A. panax | CBS 482.81 | KC584549 | KC584293 | KC584417 | KC584209 | KC584128 | KC584675 |
| A. papavericola | CBS 116606 | KC584579 | KC584321 | KC584446 | FJ357310 | FJ357298 | KC584705 |
| A. penicillata | CBS 116608 | KC584572 | KC584316 | KC584440 | FJ357311 | FJ357299 | KC584698 |
| A. penicillata | CBS 116607 | KC584580 | KC584322 | KC584447 | KC584229 | KC584153 | KC584706 |
| A. perpunctulata | CBS 115267 | KC584550 | KC584294 | KC584418 | KC584210 | KC584129 | KC584676 |
| A. petroselini | CBS 112.41 | KC584551 | KC584295 | KC584419 | KC584211 | KC584130 | KC584677 |
| A. petuchovskii | M304 | KJ443079 | KJ443124 | KJ443170 | KJ443254 | KJ649616 | – |
| A. photistica | CBS 212.86 | KC584552 | KC584296 | KC584420 | KC584212 | KC584131 | KC584678 |
| A. phragmospora | CBS 274.70 | KC584595 | KC584337 | KC584462 | JN383493 | JN383474 | KC584721 |
| A. porri | CBS 116698 | KC584553 | KC584297 | KC584421 | DQ323700 | KC584132 | KC584679 |
| A. proteae | CBS 475.90 | KC584597 | KC584339 | KC584464 | AY278842 | KC584161 | KC584723 |
| A. pseudorostrata | CBS 119411 | KC584554 | KC584298 | KC584422 | JN383483 | AY562406 | KC584680 |
| A. radicina | CBS 245.67 | KC584555 | KC584299 | KC584423 | KC584213 | KC584133 | KC584681 |
| A. scirpicola | CBS 481.90 | KC584602 | KC584344 | KC584469 | KC584237 | KC584163 | KC584728 |
| A. septorioides | CBS 106.41 | KC584559 | KC584303 | KC584427 | KC584216 | KC584136 | KC584685 |
| A. septospora | CBS 109.38 | KC584620 | KC584362 | KC584487 | FJ266489 | FJ266500 | KC584747 |
| A. shukurtuzii | M307 | KJ443082 | KJ443127 | KJ443172 | KJ443257 | KJ649620 | – |
| A. simsimi | CBS 115265 | KC584560 | KC584304 | KC584428 | JF780937 | KC584137 | KC584686 |
| A. slovaca | CBS 567.66 | KC584576 | KC584319 | KC584444 | KC584226 | KC584150 | KC584702 |
| A. smyrnii | CBS 109380 | KC584561 | KC584305 | KC584429 | AF229456 | KC584138 | KC584687 |
| A. solani | CBS 116651 | KC584562 | KC584306 | KC584430 | KC584217 | KC584139 | KC584688 |
| A. soliaridae | CBS 118387 | KC584563 | KC584307 | KC584431 | KC584218 | KC584140 | KC584689 |
| A. solidaccana | CBS 118698 | KC584564 | KC584308 | KC584432 | KC584219 | KC584141 | KC584690 |
| A. sonchi | CBS 119675 | KC584565 | KC584309 | KC584433 | KC584220 | KC584142 | KC584691 |
| Alternaria sp. | CBS 175.52 | KC584577 | KC584320 | KC584445 | KC584227 | KC584151 | KC584703 |
| A. tagetica | CBS 479.81 | KC584566 | KC584310 | KC584434 | KC584221 | KC584143 | KC584692 |
| A. tellustris | CBS 538.83 | KC584598 | KC584340 | KC584465 | FJ357316 | AY562419 | KC584724 |
| A. thalictrigena | CBS 121712 | KC584568 | KC584312 | KC584436 | EU040211 | KC584144 | KC584694 |
| A. triglochinicola | CBS 119676 | KC584569 | KC584313 | KC584437 | KC584222 | KC584145 | KC584695 |
| A. vaccariae | CBS 116533 | KC584570 | KC584314 | KC584438 | KC584223 | KC584146 | KC584696 |
| A. vaccariicola | CBS 118714 | KC584571 | KC584315 | KC584439 | KC584224 | KC584147 | KC584697 |
Fig Phylogenetic tree generated by maximum parsimony analysis of combined SSU, LSU, ITS, GPDH, tef1 and RPB2 sequence data of Alternaria species. One hundred strains are included in the analyses, which comprised 4056 characters including gaps. The tree was rooted with Stemyphylium herbarium (CBS 191.86) and Pleospora tarda (CBS 714.68). The maximum parsimonious dataset consisted of 3091 constant, 852 parsimony-informative and 113 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of ten equally most parsimonious trees with a length of 4520 steps (CI = 0.335, RI 0.708, RC = 0.237, HI = 0.665) in the first tree. MP and ML bootstrap values ≥50% and Bayesian posterior probabilities ≥0.90 are shown respectively near the nodes. Ex-type strains are in bold.

No Comments