24 Apr Boeremia
Boeremia Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 36 (2010)
Background
Boeremia was established by Aveskamp et al. (2010) to accommodate phoma-like species that are morphologically similar to Phoma exigua. To date only B. lycopersici is reported to have a sexual morph (Chen et al. 2015).
Classification—Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae
Type species—Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley, in Aveskamp, Gruyter, Woudenberg, Verkley & Crous, Stud. Mycol. 65: 37 (2010)
Distribution—Worldwide
Disease Symptoms—Black node, Bulb rot, Canker, Leaf spots, Stem rot, Shoot dieback, Tan spot
All foliar parts of a plant can be affected. Dark brown sunken lesions form at the base of the plant and eventually expand to girdle the stem, resulting in yellowing and wilting of the older leaves. This will lead to the death of the plant. Fruit infection starts as a water soaked lesion that progress rapidly into a sunken brown/black/gray lesion with concentric rings. Leaf lesions begin as small spots that develop into brown/gray lesions with concentric rings (Jones et al. 2011; Zhao et al. 2016).
Hosts—Amaryllidaceae, Apocynaceae, Araliaceae, Caprifoliaceae, Chenopodiaceae, Crassulaceae, Faba– ceae, Lamiaceae, Linaceae, Oleaceae, Rubiaceae, Sali– caceae, Solanaceae, Ulmaceae and Umbelliferae (Farr and Rossman 2019).
Morphological based identification and diversity
This genus is characterized by variable conidial shape, aseptate to 2-septate conidia in the asexual morph and a sexual morph with ellipsoidal, 1-septate ascospores. Index Fungorum lists 13 species and 12 varieties of Boeremia exigua (Index Fungorum 2019). Naming of species of Boeremia and varieties of B. exigua is mainly based on host association (Aveskamp et al. 2010; Jayasiri et al. 2017). Some B. exigua varieties and Boeremia sp. are host- specific, while others seem to have a very broad host range. Original epithets of Boeremia (and Phoma) species and varieties were based on the hosts from which they were collected and later, characters from artificial culture media were used (Boerema et al. 2004). Molecular phylogenetics has only recently been employed to separate these taxa and this has often necessitated renaming (Aveskamp et al. 2010). Thus, nomenclature is still confused (Berner et al. 2015). Colony and conidial morphology are the primary characters to identify species within this genus. However, using these characters alone in identification can cause errors as these characters may overlap between species. Therefore, it is important to use DNA sequence data when identifying species of this genus (Chen et al. 2015, 2017; Marin-Felix et al. 2017).
Molecular based identification and diversity
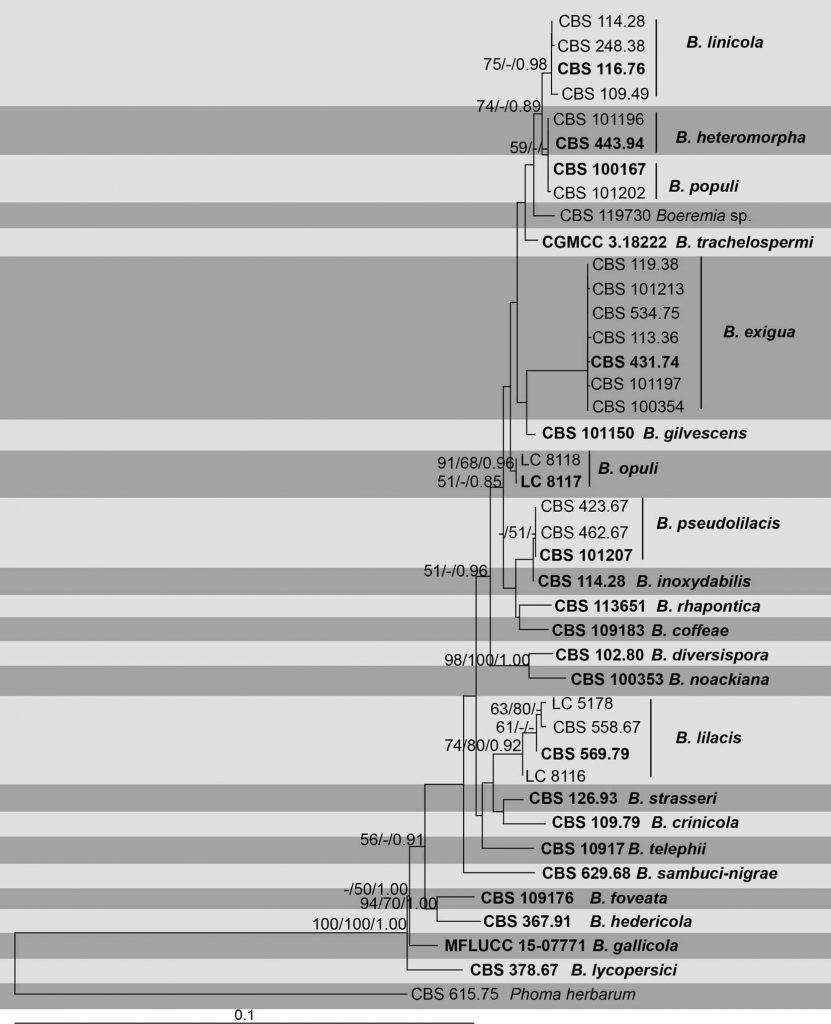
Recent studies on the taxonomy of Boeremia have employed molecular methods to reveal the phylogenetic relationships among species (Aveskamp et al. 2010). Berner et al. (2015), Chen et al. (2015, 2017), Marin-Felix et al. (2017) and Jayasiri et al. (2017) revisited the genus and described new species. This study reconstructs the phylogeny using a combined LSU, RPB2, ITS, TUB2 and TEF1-a sequence dataset. In this study, we elevate the varieties of B. exigua and change the status of ten species based on combined phylogenetic analysis as well as synonymise three varieties under B. exigua. Until further collections are found, herein we propose to keep CBS 119730 as Boeremia sp. Based on the multigene concatenated phylogenies we accept 22 species in this genus.
Boeremia coffeae (Henn.) Jayasiri, Jayaward. & K.D. Hyde, comb. nov. IF555804
≡ Ascochyta coffeae Henn., Hedwigia 41: 307 (1902)
≡Boeremia exigua var. coffeae (Henn.) Aveskamp et al., Stud. Mycol. 65: 37 (2010)
Reference Strain: CBS 109183 (= reference specimen sensu Boerema et al. 2004; Aveskamp et al. 2010; Chen et al. 2015).
For a complete description see de Gruyter et al. (2002).
This species was described from leaves of coffee plants as Ascochyta coffeae. Based on phylogenetic analyses, Aveskamp et al. (2010) placed this species in the Boeremia exigua species complex. In our phylogenetic analyses based on LSU, RPB2, ITS, TUB2 and TEF1-a, the reference strain of this species is closely related with the type strain of B. rhapontica (Fig. 2). However, B. coffeae can be differentiated from B. rhapontica by smaller pycnidia (70–80 lm vs 4–6 lm) (de Gruyter et al. 2002; Berner et al. 2015). Aveskamp et al. (2010) listed two strains under this species, CBS 119730 and CBS 109183 even though they do not cluster together in the combined multigene analysis of LSU, ITS and TUB2. CBS 119730 strain was introduced by Stewart (1957) as Ascochyta tarda, the causal agent of coffee leaf blight and stem die- back. In the combined multigene analyses of Jayasiri et al. (2017) and this study the above mentioned two strains do not cluster together. When we compared the base pair differences among these two strains, we found that CBS 119730 has 1 bp, 9 bp and 5 bp differences in ITS, RPB2 and TUB2 regions respectively. CBS 119730 differs from B. coffeae in having larger pycnidia (70–110 lm vs 70–80 lm) and conidia (9–14 lm vs 50–199 lm). According to the original description, CBS 119730 has been observed to occur together with a Mycosphaerella sp., however, the connection between these species have not yet been proven. Therefore, here we assign CBS 109183 as the reference strain (= reference specimen sensu Ariyawansa et al. 2014) for this species and keep CBS 119730 as Boeremia sp. until further collection is found.
Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley, Aveskamp et al., Stud. Mycol. 65: 37 (2010) IF515624
≡ Phoma exigua Desm. Annls Sci Nat Bot se´r 3 11(2): 282(1849)
= Boeremia exigua var. forsythia (Sacc.) Aveskamp, Gruyter & Verkley, Aveskamp et al., Stud. Mycol. 65: 37 (2010)
= Boeremia exigua var. viburni (Roum. ex. Sacc.) Aveskamp, Gruyter & Verkley, Aveskamp et al., Stud. Mycol. 65: 37 (2010)
Reference Strain: CBS 431.74 (= reference specimen sensu Boerema et al. 2004; Aveskamp et al. 2010; Chen et al. 2015).
For a complete description see Aveskamp et al. (2010).
This is the type species of the genus Boeremia. Boerema et al. (2004) assigned a reference strain (= reference specimen sensu Ariyawansa et al. 2014) for this species as CBS 431.74 and this was followed in the studies of Aveskamp et al. (2010), Chen et al. (2017) and Jayasiri et al. (2017). In the multigene phylogenetic analyses of this study, B. exigua var. forsythia and B. exigua var. vibruni clustered together with the reference strain of B. exigua (CBS 431.74). Sequences of B. exigua (CBS 431.74) and B. exigua var. forsythia (CBS 101213) are almost identical, with only 1 bp difference in each ITS and RPB2 gene regions. Between B. exigua and B. exigua var. vibruni (CBS 100354) we found only 1 bp difference in the RPB2 region. Therefore, in this study we synonymise B. exigua var. forsythia and B. exigua var. vibruni under B. exigua.
Boeremia gilvescens (Aveskamp et al.) Jayaward., Jayasiri & K.D. Hyde, comb. nov. stat. nov. IF555805
≡Boeremia exigua var. gilvescens Aveskamp, Gruyter & Verkley, Aveskamp et al., Stud. Mycol. 65: 37 (2010)
Holotype: The Netherlands, Baarn, from leaves of Dactylis purpurea, 1970, H.A. van der Aa, CBS H-16281, ex-type culture CBS 761.70.
For a complete description see Aveskamp et al. (2010).
This species differs from B. exigua by having yellowish conidial matrix and absence of positive reaction to NaOH (Aveskamp et al. 2010). LSU, ITS and TUB2 sequences of B. exigua (CBS 471.34) and B. gilvescens (CBS 761.70) are almost identical. There are only 2 bp differences in RPB2. However, there are 41 bp differences in TEF1-a gene region.
Boeremia heteromorpha (Schulzer & Sacc.) Jayaward., Jayasiri & K.D. Hyde, comb. nov. IF555806
≡Phoma heteromorpha Schulzer & Sacc., Revue mycol., Toulouse 6(22): 74 (1884)
≡Phoma exigua var. heteromorpha (Schulzer & Sacc.) Noordel. & Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 166: 109 (1989)
≡Boeremia exigua var. heteromorpha (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, Aveskamp et al., Stud. Mycol. 65: 37 (2010)
Neotype: Italy, Perugia, from Nerium oleander (Apocy– naceae), deposited in CBS Aug. 1994, A. Zazzerini, HMAS246695; ex-neotype culture CBS 443.94.
For a complete description see Chen et al. (2015).
Chen et al. (2015) designate a neotype for Phoma heteromorpha. In the phylogenetic analyses of Chen et al. (2015) and in this paper (Fig.), this species clusters with B. populi and we were unable to identify any base pair differences among these two species. However, Chen et al. (2015) maintained these as two separate species as B. heteromorpha occurred on Nerium oleander and B. populi on Populus and Salix sp. respectively. Therefore, in this study we follow Chen et al. (2015) and maintain B. heteromorpha and B. populi as two distinct species.
Boeremia inoxydabilis (Boerema & Vegh) Jayaward., Jaysiri & K.D. Hyde, comb. nov. IF555812
= Phoma exigua var. inoxydabilis Boerema & Vegh, in Vegh et al., Bull. Trimest. Soc. Mycol. Fr. 90(2): 130(1974)
Reference Strain: CBS 372.75/ATCC 32161.
Phoma exigua var. inoxydabilis was introduced to accommodate a taxon found on Vinca minor and V. major in Europe and North America (Vegh et al. 1974). Van der Aa (1973) mentioned that the French type culture PC 2198 has been lost. Aveskamp et al. (2010) mentioned that this variety may be identical to B. gilvescens. However, CBS 372.75 is closely related to B. pseudolilacis in the phylogenetic analyses (Fig). The LSU sequence of strain CBS 372.75 is short and is identical with B. gilvescens and B. pseudolilacis. The same can be seen in the ITS gene region. There is one base pair difference in the TUB2 locus among these three strains. The TEF1-α sequence of CBS 372.75 is almost identical with B. pseudolilacis. There are 25 bp differences with B. gilvescens which makes it a different species from B. gilvescens. Here we elevate this taxon to B. inoxydabilis.
Boeremia lilacis (Sacc.) Qian Chen & L. Cai, in Chen et al. Stud. Mycol. 82:170 (2015) IF515629
≡Phoma herbarum f. lilacis Sacc., Michelia 2(1):93 (1880)
≡Phoma exigua var. lilacis (Sacc.) Boerema, Phy- topath. Mediterr. 18:106 (1979)
≡Boeremia exigua var. lilacis (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, in Aveskamp et al., Stud. Mycol. 65: 38 (2010)
Reference Strain: CBS 569.79 (= reference specimen sensu Berner et al. 2015; Chen et al. 2015).
For a complete description see Chen et al. (2015).
Chen et al. (2015) elevated this taxon to species level based on the multigene phylogeny in Berner et al. (2015). In our multigene phylogenetic analysis we identified CBS 588.67, CBS 569.79, LC 8116 and LC 5178 as B. lilacis. However, CBS 588.67, LC 8116 and LC 5178 lack TEF1- α sequence data and the LSU sequences are shorter (964 bp) than the type strain CBS 569.79. This may be the reason for these four strains do not cluster together. However, further collection and sequence data are needed for clarification. Until then, we treat CBS 588.67, LC 8116 and LC 5178 as B. lilacis.
Boeremia linicola (Naumov & Vassilijevsky) Jayaward., Jayasiri & K.D. Hyde comb. nov. IF555807
≡Ascochyta linicola Namov & Vassilijecsky, Mater.
Mycol. Phytopath. Leningrad 5: 3 (1926)
≡Boeremia exigua var. linicola (Naumov & Vassili- jevsky) Aveskamp, Gruyter & Verkley, in Aveskamp et al., Stud. Mycol. 65: 39 (2010)
Reference Strain: CBS 116.76 (= reference specimen sensu Van der Aa et al. 1973; Boerema et al. 2004; Aveskamp et al. 2010; Chen et al. 2015).
The strains CBS 109.49, CBS 114.28 and CBS 248.38 clustered together with the representative strain CBS 116.76 of B. exigua var. linicola assigned by Van der Aa et al. (1973) in our phylogenetic analyses (Fig). The multigene loci sequence data for these four strains are almost identical. Here we elevate B. exigua var. linicola to B. linicola and assign CBS 116.76 as the reference specimen for this species.
Boeremia opuli (Qian Chen, Crous & L. Cai) Jayaward., Jayasiri & K.D. Hyde, comb. nov. stat. nov. IF555809
≡Boeremia exigua var. opuli Qian Chen, Crous & L. Cai, in Chen et al., Stud. Mycol. 87: 128 (2017)
Holotype: USA, from seedlings of Viburmum opulus (Caprifoliaceae), 2014, W.J Duan, HMAS 247147, ex-type culture CGMCC 3.18354.
For a complete description see Chen et al. (2017).
Chen et al. (2017) introduced this variety based on multigene analyses and morphological characters. Herein we elevate this taxon to species level as B. opuli. Morphologically this species can be distinguished based on larger pycnidia (Chen et al. 2017). It is phylogenetically closely related to B. exigua and B. gilvescens (Fig ) and differs in nine positions and eight positions in the RPB2 locus respectively.
Boeremia populi (Gruyter & P. Scheer) Jayaward., Jayasiri & K.D. Hyde, comb. nov. stat. nov. IF555810
≡Phoma exigua var. populi Gruyter & P. Scheer, J. Phytopath. 146(8–9): 413 (1998)
≡Boeremia exigua var. populi (Gruyter & P. Scheer) Aveskamp, Gruyter & Verkley, in Aveskamp et al., Stud. Mycol. 65: 39 (2010)
Holotype: The Netherlands, Deil, from a twig of Populus (×) euramericana cv. Robusta (Salicaceae), Feb 1993, A.J.P. Oort, L 995.263.325, ex-type culture CBS 100167.
For a complete description see de Gruyter et al. (2002).
Boeremia exigua var. populi is known from Populus and Salix sp. (Aveskamp et al. 2010). Herein we elevate this variety to species level and introduce B. populi. In the phylogenetic analyses of Chen et al. (2015) (based on four gene regions) and in this paper (based on five gene regions), the type strain clusters with B. heteromorpha and we were unable to identify any base pair differences among these two species. Following Chen et al. (2015) and based on the host association of these two species and until further collections are done, herein we maintain B. populi as a distinct species from B. heteromorpha.
Boeremia pseudolilacis (Aveskamp, Gruyter & Verkley), Jayaward., Jayasiri & K.D. Hyde, comb. nov. stat. nov. IF555811
≡Boeremia exigua var. pseudolilacis Aveskamp, Gruyter & Verkley, in Aveskamp et al., Stud. Mycol. 65: 39 (2010)
Holotype: The Netherlands, near Boskoop, from Syringa vulgaris (Oleaceae), 1994, J. de Gruyter, CBS H-20371, ex-type culture CBS 101207.
For a complete description see Aveskamp et al. (2010).
Boeremia exigua var. pseudolilacis was introduced by Aveskamp et al. (2010), based on morphological and phylogenetic support. Herein we raise the status to B. pseudolilacis to accommodate this taxon. This species can be identified from other Boeremia species on the basis of DAF and AFLP analyses (Aveskamp et al. 2009, 2010).
Boeremia rhapontica (Berner, Woudenb. & Tunali) Jayaward., Jayasiri & K.D. Hyde, comb. nov. stat. nov. IF555808
≡Boeremia exigua var. rhapontica Berner, Woudenb. & Tunali, in Berner et al., Biological Control 81: 70 (2014) Holotype: Turkey, from Rhaponticum repens (Asteraceae), 2002, D. Berner, BPI 843350; ex-type culture CBS 113651. For a complete description see Berner et al. (2015).
Boeremia exigua var. rhapontica was introduced by Berner et al. (2015) to accommodate the pathogen on Rhaponticum repens. Herein we elevate this to the species level and introduce B. rhapontica (for phylogenetic differences please see notes under B. coffeae).
Recommended genetic markers (Genus level) –LSU and ITS
Recommended genetic markers (Species level)—RPB2, TUB2, TEF1-a
Accepted number of species: Twenty two species
References: Boerema et al. (2004) (morphology and pathogenicity), Aveskamp et al. (2010), Chen et al. (2015, 2017), Jayasiri et al. (2017) (morphology and phylogeny), Berner et al. (2015) (morphology, pathogenicity and phylogeny).
Fig. Phylogenetic tree inferred from a maximum likelihood analysis based on analyses of a concatenated alignment of LSU, ITS, RPB2, TUB and TEF1-a sequence data of 41 strains representing the genus Boeremia, which comprise 2746 characters including gaps. Tree is rooted with Phoma herbarum (CBS 615.75). Tree topology of the ML analysis was similar to the MP and BI (not shown). The best scoring RAxML tree with a final likelihood value of -8078.423038 is presented. The matrix had 393 distinct alignment patterns, with19.32% of undetermined characters or gaps. Estimated base frequencies were as follows; A = 0.238158, C = 0.240395, G = 0.268922, T = 0.252525; substitution rates AC = 1.084080, AG = 2.823706, AT = 1.555532, CG = 0.936952, CT = 8.695282, GT = 1.000000;gamma distribution shape parameter a = 0.1000000000. The RAxML and MP bootstrap support values above 50% and Bayesian posterior probabilities above 0.90 are given near to each branch. The scale bar indicates 0.1 changes per site. The ex-type strains are in bold.
Table Boeremia. Details of the isolates used in the phylogenetic analyses
| Species | Isolate | LSU | ITS | RPB2 | TUB2 | TEF1- α |
| Boeremia coffeae | CBS 109183; PD 2000/10506; IMI 300060 | GU237943 | GU237748 | KT389566 | GU237505 | KY484678 |
| B. crinicola | CBS 109.79 | GU237927 | GU237737 | KT389563 | GU237489 | – |
| B. diversispora | CBS 102.80 | GU237930 | GU237725 | KT389565 | GU237492 | KY484676 |
| B. exigua | CBS 431.74; PD 74/2447 | EU754183 | FJ427001 | KT389569 | FJ427112 | KY484687 |
| CBS 119.38 | KT389707 | KT389490 | – | KT389784 | – | |
| CBS 113.36 | MH867235 | KY484642 | – | KY484742 | KY484683 | |
| CBS 534.75 | MH872718 | MH860950 | – | KY484746 | KY484689 | |
| CBS 101197 | GU237931 | GU237718 | KT389570 | GU237493 | KY484690 | |
| CBS 101213; PD 92/959 | GU237932 | GU237723 | KT389571 | GU237494 | KY484692 | |
| CBS 100354; PD 83/448 | GU237944 | GU237711 | KT389577 | GU237506 | – | |
| B. foveata | CBS 109176; PD 94/1394 | GU237946 | GU237742 | KT389578 | GU237508 | KY484714 |
| B. galiicola | MFLUCC 15-0771* | KX698026 | KX698037 | – | KX698030 | – |
| B. gilvescens | CBS 101150*; PD 79/118 | EU754182 | GU237715 | KT389568 | GU237495 | KY484694 |
| B. hedericola | CBS 367.91*; PD 87/229 | GU237949 | GU237842 | KT389579 | GU237511 | KY484718 |
| B. heteromorpha | CBS 443.94* | GU237935 | GU237866 | KT389573 | GU237497 | KY484700 |
| CBS 101196; PD 79/176 | GU237934 | GU237717 | KT389572 | GU237496 | KY484699 | |
| B. inoxydabilis | CBS 372.75 | MH872672 | KY484656 | – | KY484754 | KY484701 |
| B. lilacis | CBS 569.79; PD 72/741;IMI 331909 | GU237936 | GU237892 | – | GU237498 | KY484721 |
| CBS 588.67 | KT389709 | KT389492 | – | KT389786 | – | |
| LC 5178 | KY742201 | KY742047 | – | KY742289 | – | |
| LC 8116 | KY742202 | KY742048 | – | KY742290 | – | |
| B. linicola | CBS 116.76*; ATCC 32332;IMI 197074; PD 75/544 | GU237938 | GU237754 | KT389574 | GU237500 | KY484705 |
| CBS 109.49 | MH867998 | MH856453 | – | KY484755 | KY484702 | |
| CBS 248.38 | KT389703 | KT389486 | KT389575 | KT389780 | – | |
| CBS 114.28 | GU237937 | GU237752 | – | GU237499 | KY484704 | |
| B. lycopersici | CBS 378.67; PD 67/276 | GU237950 | GU237848 | KT389580 | GU237512 | KY484726 |
| B. noackiana | CBS 100353; PD 87/718 | GU237952 | GU237710 | – | GU237514 | KY484727 |
| B. opuli | LC 8117* | KY742199 | KY742045 | KY742133 | KY742287 | – |
| LC 8118 | KY742200 | KY742046 | KY742134 | KY742288 | – | |
| B. populi | CBS 100167*; PD 93/217 | GU237939 | GU237707 | – | GU237501 | KY484706 |
| CBS 101202 | KY742199 | KY742046 | KY742133 | KY742287 | KY484709 | |
| B. pseudolilacis | CBS 101207*; PD 94/614 | GU237941 | GU237721 | – | GU237503 | KY484710 |
| CBS 462.67 | KT389705 | KT389488 | – | KT389782 | – | |
| CBS 423.67 | KT389704 | KT389487 | KT389576 | KT389781 | – | |
| B. rhapontica | CBS 113651* | – | KY484662 | – | KY484760 | KY484713 |
| B. sambuci–nigrae | CBS 629.68*; CECT 20048;IMI 331913; PD 67/753 | GU237955 | GU237897 | – | GU237517 | KY484734 |
| B. strasseri | CBS 126.93; PD 73/642 | GU237956 | GU237773 | KT389584 | GU237518 | KY484735 |
| B. telephii | CBS 109175; PD 79/524 | GU237958 | GU237741 | KT389585 | GU237520 | KY484737 |
| B. trachelospermi | CGMCC 3.18222* | KY064032 | KY064028 | KY064033 | KY064051 | – |
| Boeremia sp. | CBS 119730 | GU237942 | GU237759 | KT389567 | GU237504 | – |
| Phoma herbarum | CBS 615.75 | EU754186 | FJ427022 | KP330420 | KF252703 | KR184186 |
Ex-type (ex-epitype) strains are in bold and marked with an * and reference strains are in bold

No Comments