20 Sep Phyllosticta
Phyllosticta Pers., Traité champ. (Paris): 55, 147 (1818)
Background
Phyllosticta is an important coelomycetous genus of plant pathogens known to cause diseases in a wide range of host plants worldwide. Examples include citrus black spot, black rot of grapevines and banana freckle, which cause severe economic damage to their hosts (Baayen et al. 2002; Pu et al. 2008; Glienke et al. 2011; Wikee et. al 2013 a, b, c). Some species have been reported as endophytes, saprobes or biocontrol agents. Species identification in Phyllosticta has historically been based on morphology, culture characters and host association. In recent decades molecular data have improved the knowledge of species relationships and taxonomic classifications and are expected to reveal novel cryptic species in some of the complex groups of Phyllosticta (Wikee et. al 2013a, b, c).
Phyllosticta was introduced by Persoon (1818) and typified by P. convallariae Pers. Since then numerous species have been added to the genus and 3215 names are listed under Phyllosticta in Index Fungorum (30 Jan 2018). Sexual morphs are in Guignardia with 344 species names listed in Index Fungorum (30 Jan 2018). Following the introduction of the one-fungus one-name rule, Phyllosticta (1818) was adopted as the correct name for this genus because it is older than Guignardia (1892) and names in Guignardia should be made synonyms of Phyllosticta (Sultan et al. 2011; Wikee et al 2011, 2013a,b,c ).
Classification – Dothideomycetes, incertae sedis, Botryosphaeriales, Phyllostictaceae
Type species – Phyllosticta convallariae Pers, Traité champ. (Paris): 148 (1818)
Distribution – Worldwide
Disease Symptoms – Normally Phyllosticta species cause small necrotic leaf lesions that are circular, brown to dark brown or sometimes reddish at the margin. Pycnidia can be found on the lesions and are usually black, globose to subglobose and semi immersed. After infection, the leaf may become dry in the centre of the lesion and the infected tissue falls out leaving a hole (Glienke et al. 2011). When freckle disease occurs on banana species, pycnidia and ascomata formed on fruits give the lesion a sandpaper texture. Leaves of banana turn yellow when infected with this Phyllosticta (Wikee et. al 2013a).
Hosts – Phyllosticta species are mostly plant pathogens causing diseases in fruits and leaf spots on a broad range of host plants including economically important crops and ornamentals such as citrus, banana, apple, grapes, cranberry, orchids, Ficus sp., Buxus sp. and maple amongst many others (Baayen et al. 2002; Glienke et al. 2011; Wikee et. al 2013a).
Morphological based identification and diversity
This genus has undergone many significant changes since its introduction. Phyllosticta species were considered to be Phoma-like foliar pathogens. On other plant parts, Phyllosticta was regarded as a parasite and Phoma as a saprobe or wound parasite. The first monograph on Phyllosticta sensu stricto was by van der Aa (1973) using material collected in Europe and North America. He described and illustrated 46 species, and listed the sexual morphs for twelve species and the spermatial morphs for 17 species. In 2002 van der Aa & Vanev further revised the species in Phyllosticta and accepted 190 epithets (Wikee et al. 2013).
Schoch et al. (2006) placed Phyllosticta in Botryosphaeriaceae in order Botryosphaeriales and this was accepted by Crous et al. (2006) and Liu et al. (2012). The family Phyllostictaceae (as Phyllostictei) was first proposed by Fries (1849). This family name was re-instated by Wikee et al (2013) who revealed that it is sister to Botryosphaeriaceae.
Species in Phyllosticta are recognised by the production of pycnidia containing aseptate, hyaline, ovoid to ellipsoid or cylindrical conidia with a single apical appendage and covered by a mucous layer (van der Aa 1973; Wikee et al 2013a). However, some Phyllosticta species, such as P. colocasiicola, P. minima, and P. sphaeropsoidea do not have an appendage or mucus layer. Furthermore, these mucoid appendages may vary in size and shape according to the media on which the culture is grown, and sometimes with time, it may disappear. Pycnidia are usually globose to subglobose, unilocular and closely connected on a subepidermal pseudostroma. Ascomata are globose to pyriform, unilocular with a central ostiole and erumpent through the host epidermis. There is a thin peridium with a wall comprising a few layers of angular cells. Asci are 8 spored, bitunicate, clavate to broadly ellipsoid with a wide, obtusely rounded apex and tapering gradually to a small pedicel and with a well-developed ocular chamber. Ascospores are hyaline, aseptate, ellipsoid to limoniform, usually with mucilaginous caps and often surrounded by a mucilaginous sheath, sometimes slightly elongated and often multiguttulate or with a large single central guttule (van der Aa 1973; Wikee et al 2013a). However, species cannot be identified reliably on the basis of morphological characters alone due to plasticity and overlapping of dimensions.
Molecular based identification and diversity
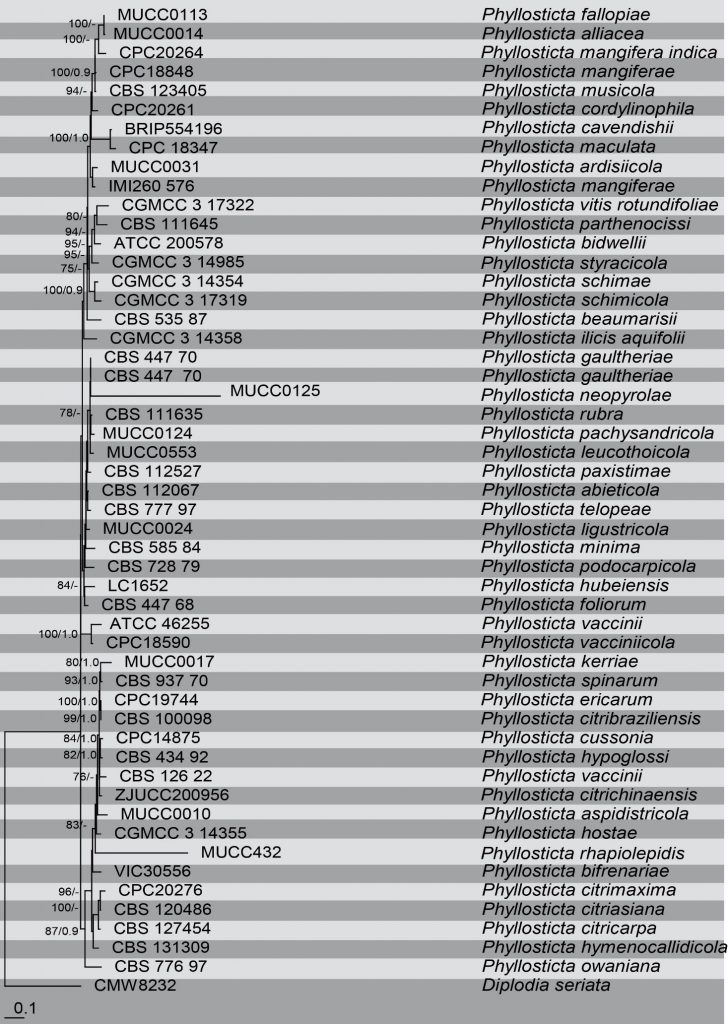
Molecular methods have been used in taxonomic studies of Phyllosticta to reveal phylogenetic relationships between the species and also to resolve species complexes within the genus (Wulandari et al. 2009; Glienke et al. 2011; Wikee et al.2011). Combined DNA phylogenetic analysis based on ITS, intron-dominated loci of genes like TEF1- α, ACT and more conserved gene regions such as LSU and GAPDH are used to reconstruct the phylogenetic relationships between the species. We reconstruct the phylogeny of the genus Phyllosticta (Fig) based on analyses of a combined ITS, TEF1- α, ACT, LSU and GAPDH sequence data. It contains recently introduced species and corresponds to previous studies.
Recommended genetic markers (genus level) – ITS
Recommended genetic markers (species level) – ITS, LSU, TEF, GAPDH and ACT
Accepted number of species: There are 3208 species epithets in Index Fungorum (2018) under this genus, but only 190 are currently accepted.
References: Wulandari et al. 2009 (pathogens), Glienke et al. 2011 (taxonomy), Wikee et al. 2011, 2013a, b (review), Su and Cai 2012 (Phylogeny), Hyde et al. 2013 (taxonomy, phylogeny), Kirk et al. 2013 (genus accepted), Slippers et al. 2013 (phylogeny), Wijayawardene et al. 2014c (Outline, phylogeny), Wu et al. 2014c (species in banana).
Table. Phyllosticta. Details of the isolates used in the phylogenetic analyses. Ex-type (ex-epitype) strains are in bold and marked with an * and voucher strains are in bold.
| Species | Isolate | ITS | LSU | TEF-I | ACT | GAPDH |
| P. abieticola | CBS112067* | KF170306 | EU754193 | – | KF289238 | – |
| P. alliacea | MUCC0014* | AB454263 | – | – | – | – |
| P. ampelicida | ATCC200578* | KC193586 | – | – | KC193581 | KC193584 |
| P. ardisiicola | NBRC102261* | AB454274 | – | – | – | – |
| P. aspidistricola | NBRC102244* | AB454260 | – | – | – | – |
| P. beaumarisii | CBS 535.87* | AY042927 | KF306229 | KF289170 | KF306232 | KF289074 |
| P. bifrenariae | CBS 128855* | JF343565 | KF206209 | JF343586 | JF343649 | JF433744 |
| P. capitalensis | IMI 260.576* | JF261459 | KF206222 | JF261501 | JF343641 | JF343748 |
| P. capitalensis | CPC 18848* | JF261465 | KF206255 | JF261507 | KF289289 | JF343776 |
| P. cavendishii | BRIP554196* | JQ743562 | – | KF009743 | KF014080 | – |
| P. citriasiana | CBS 120486* | FJ538360 | KF206314 | FJ538418 | FJ538476 | JF343686 |
| P. citribraziliensis | CBS 100098* | FJ538352 | KF206221 | FJ538410 | FJ538468 | JF343691 |
| P. citricarpa | CBS 127454* | JF343583 | KF206306 | JF343604 | JF343667 | JF343771 |
| P. citrichinaensis | ZJUCC 200956* | JN791620 | – | JN791459 | JN791533 | – |
| P. citrimaxima | CPC 20276* | KF170304 | KF206229 | KF289222 | KF289300 | KF289157 |
| P. concentric | CBS 937.70* | FJ538350 | KF206291 | FJ538408 | KF289257 | JF411745 |
| P. cordylinophila | CPC 20261* | KF170287 | KF206242 | KF289172 | KF289295 | KF289076 |
| P. cussonia | CPC 14875* | JF343579 | KF206278 | JF343600 | JF343663 | JF343765 |
| P. dendrobii | CGMCC 3.18666* | MF180193 | MF180210 | MF180202 | MF180220 | MF180229 |
| P. elongate | CBS 126.22* | FJ538353 | – | FJ538411 | FJ538469 | KF289164 |
| P. ericarum | CBS 132534* | KF206170 | KF206253 | KF289227 | KF289291 | KF289162 |
| P. fallopiae | MUCC0113* | AB454307 | – | – | – | – |
| P. foliorum | CBS 447.68* | KF170309 | KF206287 | KF289201 | KF289247 | KF289132 |
| P. gaultheriae | CBS 447.70* | JN692543 | KF206298 | JN692531 | KF289248 | JN692508 |
| P. gaultheriae | CBS 447.70* | JN692543 | KF206298 | JN692531 | KF289248 | JN692508 |
| P. hostae | CGMCC 3.14355* | JN692535 | – | JN692524 | JN692512 | JN692504 |
| P. hubeiensis | CGMCC 3.14986* | JX025037 | – | JX025042 | JX025032 | JX025027 |
| P. hymenocallidicola | CBS 131309* | JQ044423 | JQ044443 | KF289211 | KF289242 | KF289142 |
| P. hypoglossi | CBS 434.92* | FJ538367 | KF206299 | FJ538425 | FJ538483 | JF343695 |
| P. ilicis-aquifolii | CGMCC 3.14358* | JN692538 | – | JN692526 | JN692514 | – |
| P. illicii | CGMCC 3.18670* | MF180195 | MF180212 | MF180203 | MF180221 | – |
| P. kerriae | MAFF240047* | AB454266 | – | – | – | – |
| P. leucothoicola | CBS 136073* | AB454370 | AB454370 | – | KF289310 | – |
| P. ligustricola | MUCC0024* | AB454269 | – | – | AB704212 | – |
| P. maculate | CPC18347* | JP743570 | – | KF009700 | KF014016 | – |
| P. mangifera-indica | CPC 20274* | KF170305 | KF206240 | KF289190 | KF289296 | KF289121 |
| P. minima | CBS 585.84* | KF206176 | KF206286 | KF289204 | KF289249 | KF289135 |
| P. musicola | CBS123405* | FJ538334 | – | FJ538392 | FJ538450 | – |
| P. neopyrolae | CPC 21879* | AB454318 | AB454241 | – | AB704233 | – |
| P. owaniana | CBS 776.97* | KJ538368 | KF206293 | FJ538426 | KF289254 | JF343767 |
| P. pachysandricola | MUCC 124* | AB454317 | AB454317 | – | AB704232 | – |
| P. parthenocissi | CBS111645* | EU683672 | – | JN692530 | JN692518 | – |
| P. paxistimae | CBS 112527* | KF206172 | KF206320 | KF289209 | KF289239 | KF289140 |
| P. podocarpicola | CBS 728.79* | KF206173 | KF206295 | KF289203 | KF289252 | KF289134 |
| P. rhaphiolepidis | MUCC 432* | DQ632660 | – | – | AB704242 | – |
| P. rubra | CBS 111635* | KF206171 | EU754194 | KF289198 | KF289233 | KF289129 |
| P. schimae | CGMCC 3.14354* | JN692534 | – | JN692522 | JN692510 | JN692506 |
| P. schimicola | CGMCC 3.17319* | KJ847426 | – | KJ847448 | KJ847434 | KJ854895 |
| P. styracicola | CGMCC 3.14985* | JX052040 | – | JX025045 | JX025036 | KF289141 |
| P. telopeae | CBS 777.97* | KF206205 | KF766384 | KF289210 | KF289255 | KF289141 |
| P. vaccinii | ATCC 46255* | NR147339 | – | KC193582 | KC193580
|
KC193583 |
| P. vacciniicola | CPC18590* | – | KF206257 | KF289229 | KF289287 | KF289165 |
| P. vitis-rotundifoliae | CGMCC 3.17322* | KJ847428 | – | KJ847450 | KJ847436 | KJ847442 |
| Diplodia seriata | CMW8232 | AY972105 | – | DQ280419 | AY972111 | – |
Fig. Phylogenetic tree generated by maximum likelihood analysis of combined ITS, TEF1, ACT, LSU and GPDH sequence data of Phyllosticta species. Sequences were obtained from GenBank. Fifty-four strains are included in the analyses, which comprise 2739 characters including gaps. Single gene analyses were carried out to compare the topology of the tree and clade stability. The tree was rooted with Diplodia seriata (CMW8232) Tree topology of the Bayesian analysis was similar to the RAxML. The best scoring RAxML tree with a final likelihood value of = -18593.839155 is presented. The matrix had 1110 distinct alignment patterns, with 37.85%of undetermined characters or gaps. Estimated base frequencies were as follows; A = 0.209468, C = 0.292249, G = 0.275727, T = 0.222557; substitution rates AC = 1.099701, AG = 2.944335, AT = 1.274132, CG = 1.149823, CT = 6.450643, GT = 1.000000; gamma distribution shape parameter α = 0.456374. RAxML and Bayesian posterior probabilities value ≥70% (BT) and 0.9 (PP) are shown respectively near the nodes.

No Comments