17 Sep Fomitiporia
Fomitiporia Murill, N. Amer. Fl. (New York) 9(1):7 (1907)
Background
The genus was established by Murrill (1907). The main characters of the genus include resupinate to pileate basidiocarp, hyaline and subglobose to globose, dextrinoid and cyanophilous basidiospores, dimitic hyphal and variable cystidioles and hymenial setae (Chen and Cui 2017). The genus has been divided into two groups based on morphological characters and basidiomata habit. There are species with pileate basidiomata (e.g. F. robusta, F. erecta, F. hippophaeicola) and species sharing resupinate basidiomata (e.g., F. langloisii, F. punctata, F. pseudopunctata) (Campos Santana et al. 2014). Fomitiporia are distributed worldwide and contain approximately 50 taxa plus numerous unidentified species (Vlasák and Kout 2011; Chen and Cui 2017; Morera et al. 2017). Fomitiporia species are pathogens and saprobes on numerous hardwood genera, for example F. mediterranea has been reported as the main agent for the esca-associated white heart rot in Europe and South Africa (Fischer 2002; Fischer and Kassemeyer 2003; Cloete et al. 2014). Fomitiporia australiensis, F. mediterranea, and F. punctata are associated with esca disease of grapevine (Fischer et al. 2005). Amalfi et al. (2012) introduced F. cupressicola as a parasite of living Cupressus arizonica. Disease of the Japanese pear tree (Pyrus pyrifolia var. culta) is caused by F. torreyae (Fukuta et al. 2016). Some species are an important medicinal resource e.g., F. ellipsoidea, F. hartigii, F. punctate and F. robusta (Dai et al. 2010; Zan et al. 2015; Liu et al. 2017).
Classification – Agaricomycetes, Incertae sedis, Hymenochaetales, Hymenochaetaceae
Type species – Fomitiporia langloisii Murill, N. Amer. Fl. (New York) 9(1):7(1907)
Distribution – Worldwide
Disease Symptoms – Esca disease
The initial symptoms occur on leaves as malformation and dwarfism (Fukuta et al. 2016). Dark red or white stripes occur as the foliar symptom of this disease and become yellow. Symptomatic leaves can dry completely and premature defoliation can occur. Small, circular, dark spots with a brown-purple border can be seen on fruits (Cortesi 2000). Shoots and twigs die as the damage expands to the trunk. From a cross section of the trunks and large branches, light/white coloured, rotted center, surrounded by brown hard necrotic wood can be observed (Elena et al. 2018). When the disease becomes severe, decaying of the tree can be observed (Fukuta et al. 2016).
Hosts – Occurs on many important plant families including Asteraceae, Fabaceae, Lamiaceae, Lauraceae, Moraceae, Myrtaceae, Oleaceae, Sapindaceae, Rosaceae and Vitaceae (Rajchenberg and Robledo 2013; Cloete et al. 2015)
Morphological based identification and diversity
Fiasson and Niemelä (1984) redefined Fomitiporia punctata (P. Karst.) Murrill as the representative of the genus and considered F. langloisii Murrill as a synonym of Fomitiporia punctata (P. Karst.) Murrill. However, later F. langloisii was re-established as the type species based on phylogenetic analysis and herbarium studies (Decock et al. 2007). Identification of Fomitiporia has been difficult and the species were problematic and in need of clarification. The generic status of the genus was confirmed by Fischer (1996) and Dai (1999). Multi-locus phylogenetic analysis (LSU + ITS + TEF1-α + RPB2) combined with traditional characters were used to re-examine the classification of the genus (Amalfi et al 2012; Chen and Cui 2017; Morera et al. 2017). Similarity of morphological characters, multi-gene phylogenetic approach and geographical distribution have been used to resolve classification problems in this genus. Recently, five species Fomitiporia alpina B.K. Cui & Hong Chen, F. gaoligongensis B.K. Cui & Hong Chen, F. hainaniana B.K. Cui & Hong Chen, F. subrobusta B.K. Cui & Hong Chen and F. subtropica B.K. Cui & Hong Chen were introduced from China and F. impercepta Morera, Robledo & Urcelay was described from Argentina based on multi-gene phylogenetic analysis and morphological characterization (Chen and Cui 2017; Morera et al. 2017). In addition, Liu et al. (2018) reported F. rhamnoides T. Z. Liu & F. Wu. a novel species from China. There are 81 Fomitiporia names listed in Index Fungorum (2018), however, some of them are synonyms and some were transferred to other taxa based on phylogenetic evidence. For example F. dryophila Murrill, F. earleae Murrill, F. jamaicensis Murrill, F. laminate Murrill, F. langloisii Murrill, F. lloydii Murrill., F. maxonii Murrill, F. obliquiformis Murrill and F. tsugina Murrill were synonymized under F. punctata (P. Karst.) Murrill. Fomitiporia ellipsoidea B.K. Cui & Y.C. Dai was transferred to Phellinus ellipsoideus (Cui and Decock 2013). Some species have been rearranged into Fomitiporia; example.g., Phellinus rosmarini Bernicchia has been recombined as Fomitiporia rosmarini (Bernicchia) Ghobad-Nejhad & Y.C. Dai (Ghobad-Nejhad and Dai 2007), while Phellinus spinescens J.E. Wright & G. Coelho was recombined as F. spinescens (J.E. Wright & G. Coelho) G. Coelho, Guerrero & Rajchenb. Phellinus uncinatus Rajchenb was transferred as F. uncinata (Rajchenb.) G. Coelho, Guerrero & Rajchenb. (Coelho et al 2009).
Basidiocarp and basidiospore characters can be used to identify this genus. However, due to inconsistency, cystidioles and hymenial setae cannot be used in species identification (Chen and Cui 2017; Liu et al. 2018). Therefore, use of DNA sequence data is crucial.
Molecular based identification and diversity
Classification of the genus was neglected for a long-time as Fomittiporia was considered a synonym of Phellinus (Núñez and Ryvarden analysis, Fomitiporia was confirmed to be a homogeneous genus within the Hymenochaetaceae (Zhou and Xue 2012). Recently, based on phylogenetic evidence, new species of Fomitiporia have been described and new combinations have been made into the genus (Fischer 2002; Fischer and Binder 2004; Decock et al. 2005, 2007; Fischer et al. 2005; Dai et al. 2008; Dai and Cui 2011; Amalfi et al. 2010, 2012; Zhou and Xue 2012; Amalfi and Decock 2014; Cloete et al. 2014; Campos-Santana et al. 2014; Chen et al. 2016a; Vlasak and Vlasak 2016; Li et al 2016; Chen and Cui 2017, Morera et al. 2017, Liu et al. 2018). Single gene and multigene phylogenies demonstrated that Fomitiporia is a monophyletic group (Wagner and Fischer 2001, 2002; Fischer 2002; Fischer and Binder 2004, Fischer et al. 2005, Decock et al. 2005, 2007, Larsson et al. 2006, Amalfi and Decock 2014, Campos-Santana et al. 2014). In this study we provide a phylogenetic tree based on multi-locus phylogenetic analysis (LSU + ITS + TEF1-α + RPB 2). Sequences of F. rhamnoides could not be analysed as they are unavailable in Genbank. The results from this study provide a similar topology to those obtained by Chen and Cui (2017). There is still a need for a better marker to provide better resolution in this genus.
Recommended genetic markers (Genus level) – ITS
Recommended genetic markers (Species level) – LSU, ITS, TEF1- α, RPB2
Accepted number of species: There are 81 species epithets in Index Fungorum (2018) under this genus. However, only 50 are accepted, but sequence data are only available for 46 species.
References: Fischer 2002, Fischer et al. 2005, Campos-Santana et al. 2014, Chen and Cui 2017, Morera et al. 2017 (morphology and phylogeny), Rajchenberg and Robledo 2013, Elena et al. 2018 (morphology, phylogeny and pathogenicity).
Table Fomitiporia. Details of the isolates used in the phylogenetic analyses. Ex-type (ex-epitype) strains are in bold and marked with an * and voucher strains are in bold.
| Species | Isolate | LSU | ITS | TEF1-α | RPB2 |
| Fomitiporia aethiopica | MUCL 44777* | AY618204 | GU478341 | GU461893 | JQ087956 |
| F. alpina | Dai 15735 | KX639645 | KX639627 | KX639664 | KX639680 |
| F. apiahyna | MUCL 51451 | GU461997 | GU461963 | GU461896 | JQ087958 |
| F. atlantica | FLOR 58554 | KU557526 | KU557528 | – | – |
| F. australiensis |
MUCL 49406 |
GU462001 | AY624997 | GU461897 | JQ087959 |
| F. baccharidis | MUCL 47756 | JQ087913 | JQ087886 | JQ087940 | JQ087993 |
| F. bakeri | FP-134784-Sp | JQ087901 | JQ087874 | JQ087928 | JQ087960 |
| F.bannaensis | MUCL 45926 | EF429217 | GU461942 | GU461898 | JQ087961 |
| F. calkinsii | MUCL 51095 | KF444708 | KF444685 | KF444754 | KF444731 |
| F. capensis | MUCL 53009 | JQ087917 | JQ087890 | JQ087944 | JQ087997 |
| F. castilloi | MUCL 53481* | JQ087916 | JQ087889 | JQ087943 | JQ087996 |
| F. cupressicola | MUCL 52486* | JQ087904 | JQ087877 | JQ087931 | JQ087965 |
| F. deserticola | PRM 934073 | – | KT381632 | – | – |
| F. dryophila | TJV-93-232 | EF429221 | EF429240 | GU461902 | JQ087969 |
| F. erecta | MUCL 49871 | GU461976 | GU461939 | GU461903 | JQ087971 |
| F. expansa | MUCL 55026 | KJ401032 | KJ401031 | KJ401033 | KJ401034 |
| F. fissurata | PRM922626 | – | KT381627 | – | – |
| F. gabonensis | MUCL 47576* | GU461990 | GU461971 | GU461923 | JQ087972 |
| F. gaoligongensis | Cui 8261 | KX639642 | KX639624 | KX639663 | KX639678 |
| F. hainaniana | CL06-372 | KX639654 | KX663826 | KX639660 | – |
| F. hartigii | MAFF 11–20016 | JQ087909 | JQ087882 | JQ087936 | JQ087975 |
| F. hippophaëicola | MUCL 31746 | AY618207 | GU461945 | GU461904 | JQ087976 |
| F. impercepta | CORDC00005289 | MF615266 | MF615298 | – | – |
| F. ivindoensis | MUCL 51312* | GU461978 | GU461951 | GU461906 | JQ087979 |
| F. langloisii | MUCL 46375 | EF429225 | EF429242 | GU461908 | JQ087980 |
| F. maxonii | MUCL 46017 | EF429230 | EF433559 | GU461910 | JQ087983 |
| F. mediterranea | MUCL 45670 | GU461980 | GU461954 | GU461913 | JQ087985 |
| F. neotropica | MUCL 51335* | KF444721 | KF444698 | KF444771 | KF444744 |
| F. nobilissima | MUCL 51289* | GU461984 | GU461965 | GU461920 | JQ087987 |
| F. norbulingka | Cui 9770 | KU364430 | KU364420 | KU364433 | – |
| F. pentaphylacis | Yuan 6012 | JQ003901 | JQ003900 | KX639671 | KX639683 |
| F. polymorpha | MUCL 46166 | DQ122393 | GU461955 | GU461914 | JQ087988 |
| F. pseudopunctata | MUCL 51325 | GU461981 | GU461948 | GU461916 | JQ087998 |
| F. punctata | MUCL 34101 | AY618200 | GU461947 | GU461917 | JQ088000 |
| F. punicata | Cui 23 | GU461991 | GU461974 | GU461927 | JQ088002 |
| F. robusta | CBS 389.72 | JQ087919 | JQ087892 | JQ087946 | JQ088004 |
| F. sonorae | MUCL 47689 | JQ087920 | JQ087893 | JQ087947 | JQ088006 |
| F. subhippophaëicola | Cui 12096 | KU364426 | KU364421 | KU364437 | – |
| F. subrobusta | Dai 13576 | KX639635 | KX639617 | KX639655 | KX639672 |
| F. subtilissima | FURB47557 | KU557527 | KU557531 | KU557532 | KU557533 |
| F. subtropica | Cui 9122 | KX639640 | KX639622 | KX639661 | KX639677 |
| F. tabaquilio | MUCL 46230 | DQ122394 | GU461940 | GU461931 | JQ088008 |
| F. tenuis | MUCL 44802* | AY618206 | GU461957 | GU461934 | JQ088010 |
| F. tenuitubus | Dai 16204 | KX639637 | KX639619 | KX639657 | KX639674 |
| F. texana | MUCL 47690 | JQ087921 | JQ087894 | JQ087948 | JQ088013 |
| F. torreyae | MUCL 47628 | JQ087923 | JQ087896 | JQ087950 | JQ088015 |
| F. tsugina | MUCL 52702 | JQ087925 | JQ087898 | JQ087952 | JQ088017 |
| F. rhamnoides | Dai 18091 | MH234392 | MH234389 | – | – |
| Phellinus uncisetus (out group) | MUCL 46231 | EF429235 | GU461960 | GU461937 | JQ088020 |
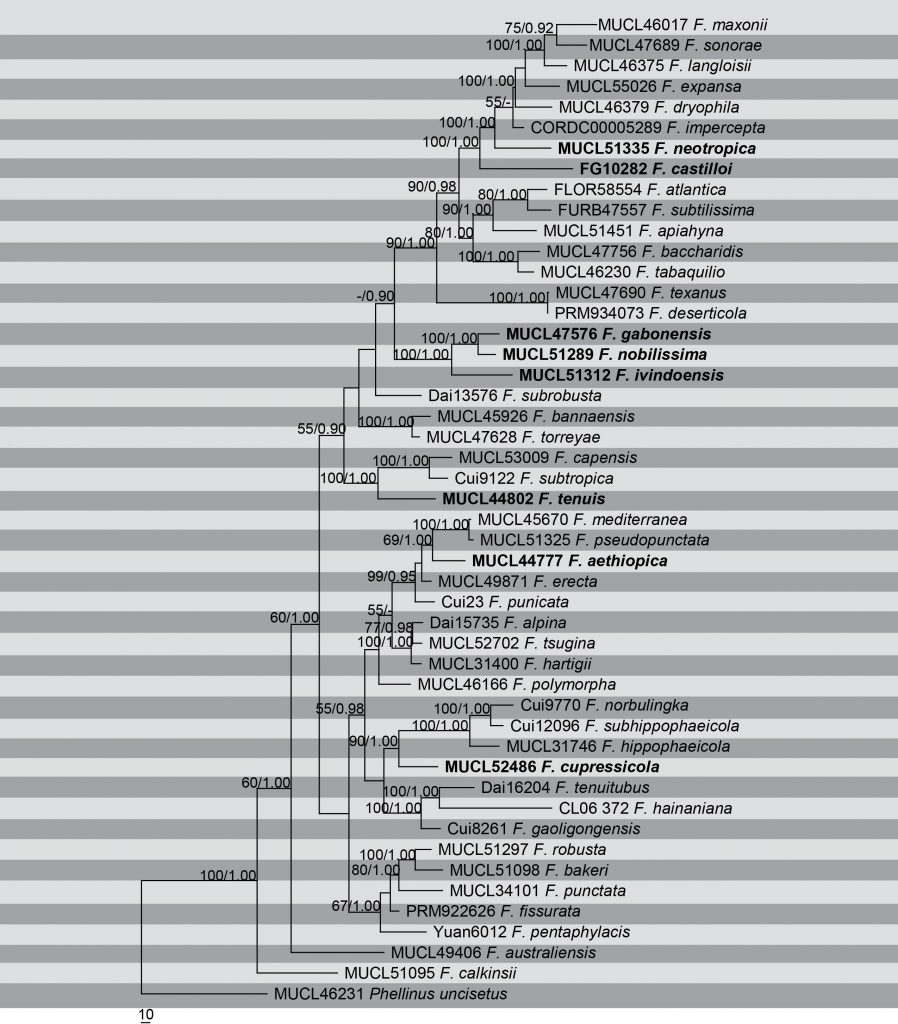
Fig. Phylogenetic tree generated by maximum Parsimony analysis of combined nLSU, ITS, TEF1-α and RPB2 sequence data of Fomitiporia species. Related sequences were obtained from GenBank. Forty five strains are included in the analyses, which comprise 3836 characters including gaps. Tree was rooted with Phellinus uncisetus (MUCL 46231). The maximum parsimonious dataset consisted of 2537 constant, 841 parsimony-informative and 458 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of six equally most parsimonious trees with a length of 3416 steps (CI = 0.511, RI =0.598, RC = 0.305, HI = 0.489) in the first tree. Bayesian posterior probabilities and MP bootstrap values ≥50% are shown respectively near the nodes. The scale bar indicates 10 changes. The ex-type strains are in bold.

No Comments