24 Oct Erysiphaceae
Erysiphaceae Tul. & C. Tul. [as ‘Erysiphei’], Select. fung. carpol. (Paris) 1: [191] (1861) Background
Powdery mildews belong to Erysiphales of Ascomycota (Mori et al. 2000). Powdery mildews are one of the most prevalent and easily recognizable of plant diseases (Glawe 2008). Mucor erysiphe, published by Linnaeus (1753), was the first binomial referring to powdery mildew (now known as Phyllactinia guttata) (Braun and Cook 2012). Infections are often conspicuous owing to the profuse production of conidia that give them their common name. Powdery mildews are also models for basic research on host-parasite interactions, developmental morphology, cytology, and molecular biology (Glawe 2008). Erysiphaceae is obligately parasitic and as such, their life cycle depends completely on living hosts, from which they obtain nutrients without killing host cells and without which they are unable to survive. As they are obligate plant pathogens, researchers have not had the advantage of routinely cultivating these taxa on artificial media. However, many powdery mildews have been grown on detached leaves of their hosts (Hirose et al. 2005). Powdery mildews seldom kill their host, but are responsible for water and nutrient loss and impaired growth and development. They can increase respiration and transpiration and interfere with photosynthesis and reduce yields.
Changes in host range directly cause the niche separation of powdery mildews and thus may become a trigger of speciation in their evolution. It is possible that studying the evolutionary history of powdery mildews will not only reveal facts on fungal evolution but may also lead us to consider the evolutionary history of angiosperm plants (Takamatsu 2004; Matsuda and Takamatsu 2003; Hirata et al. 2000; Mori et al. 2000).
The first systematic trial to identify the conidial states of powdery mildews at the species level was made by Ferraris (1910), who grouped species of Oidium according to the size and shape of their conidia and provided a key to its species. Foex (1913), Jaczewski (1927), and Brundza (1934) contributed to the classification of the conidiophore types. Jaczewski (1927) introduced the terms ‘Euoidium and Pseudoidium’ for Oidium states with catenate and solitary conidia, respectively. Yarwood (1957) provided a survey on the Erysiphaceae, including the asexual morphs. Boesewinkel (1980) provided the first comprehensive key based on a combination of more than 12 morphological characteristics observed on conidia, conidiophores, appressoria, haustoria, fibrosin bodies, and mycelium. Braun (1987) issued a second comprehensive monograph of the Erysiphales encompassing all powdery mildew taxa known at that time. Shin and La (1993) and Shin and Zheng (1998) introduced some new morphological features of taxonomic relevance. A progressive report was provided by the work of Cook et al. (1997), who examined the surface of conidia by scanning electron microscopy and separated Oidium into eight subgenera. Braun (1999) discussed the classification of Erysiphaceae as proposed by Cook et al. (1997) and introduced some corrections and alterations. Fundamental innovations in the generic taxonomy of the group based on molecular and SEM examination and a better insight into the phylogeny are results of comprehensive investigations over the last decade (Takamatsu et al. 1998, 1999, 2000, 2005a,b, 2008; Matsuda and Takamatsu 2003; Hirose et al. 2005; Liberato et al. 2006; Braun and Cook 2012).
Classification – Ascomycota, Pezizomycotina, Leotiomycetes, Leotiomycetidae, Erysiphales
Type genus – Erysiphe R. Hedw. ex DC.
Distribution – worldwide
Disease symptoms – powdery mildew
The initial signs of infection appear on young leaves in the form of small, raised blisters, which cause the leaves to curl and expose the under surfaces. As the disease progresses, round, pinpoint powdery white spots dusting the upper surfaces of leaves, as well as stems and occasionally fruiting occurs. As the disease becomes severe, the spots will become larger, and more interconnected and irregular in shape. Over time they progress from younger to older leaves and the undersides of leaves. However, mature leaves are usually much less severely infected than new or young leaves. If the white patches (which have a granular, powdery texture) are wiped away, the growths will return in a matter of days. Severely infected leaves will turn yellow, dry out and drop from the plant. Buds and growing tips of shoots can also become infected, eventually becoming distorted and stunted (Bushnell and Allen 1962; Davis et al. 2001; Romero et al. 2003; Oberti et al. 2014; Saharan et al. 2019).
Hosts– The host range of this fungal group is strictly confined to angiosperms and powdery mildews have never been reported to infect ferns or gymnosperms (Amano 1986; Hirata et al. 2000; Takamatsu et al. 2010). They affect a wide range of angiosperms such as cereals and grasses, vegetables, ornamentals, weeds, shrubs, fruit trees, and broad-leaved shade and forest trees. Powdery mildews are considered as host-specific.
Pathogen biology, disease cycle and epidemiology
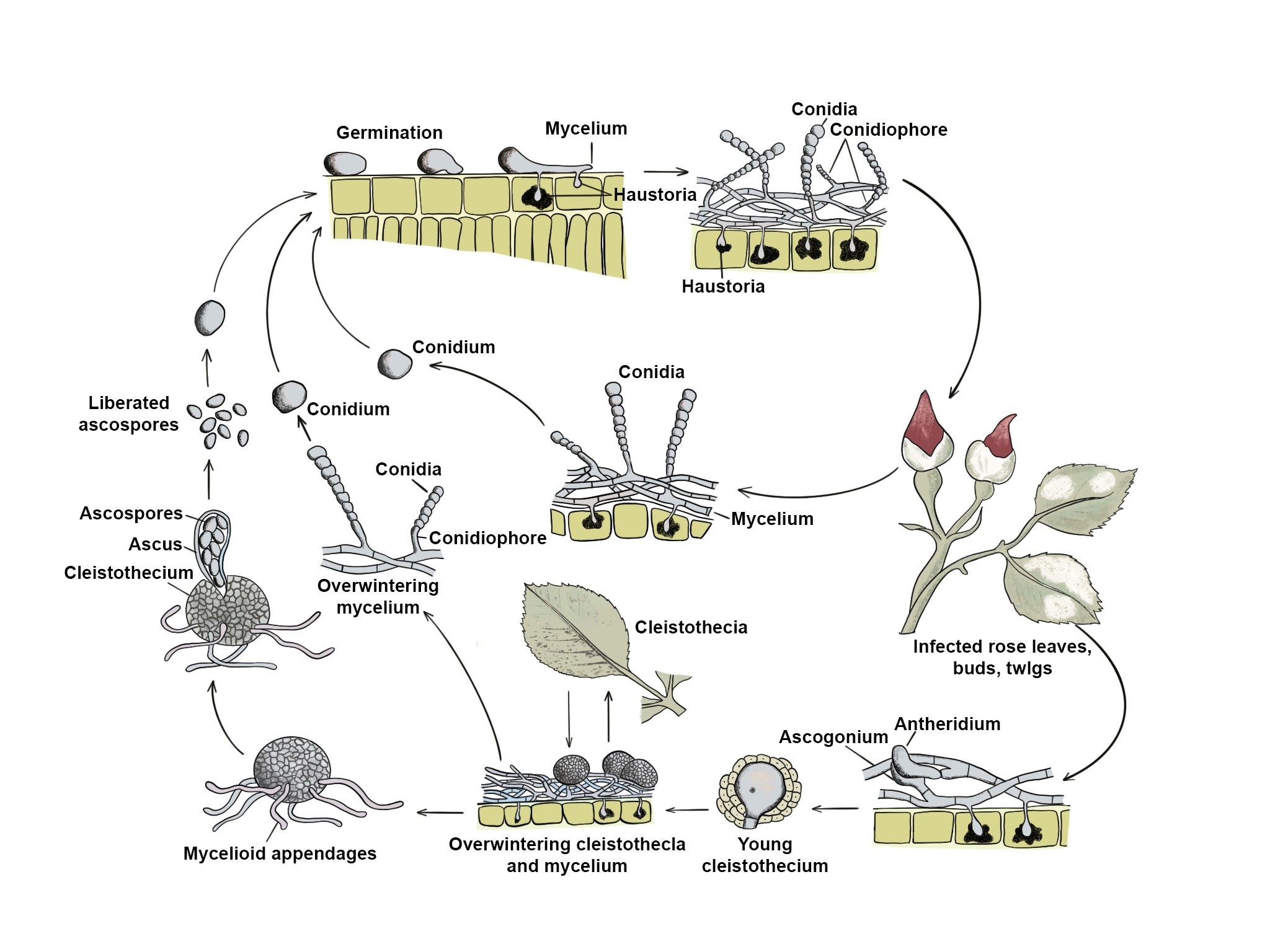
Powdery mildews tend to grow superficially, or epiphytically, on plant surfaces. During the growing season, hyphae are produced on both the upper and lower leaf surfaces, although some species are restricted to one leaf surface. Infections can also occur on stems, flowers or fruit. Specialized absorption cells, termed haustoria, extend into the plant epidermal cells to obtain nutrition. While most powdery mildews produce epiphytic mycelium, a few genera produce hyphae that are within the leaf tissue; this is known as endophytic growth. Conidia are produced on plant surfaces during the growing season. They develop either singly or in chains on conidiophores. Conidiophores arise from the epiphytic hyphae, or in the case of endophytic hyphae, the conidiophores emerge through leaf stomata. At the end of the growing season, powdery mildews produce ascospores, in a sac-like ascus enclosed in a fruiting body called a chasmothecium. The chasmothecium is generally spherical with no natural opening; asci with ascospores are released when a crack develops in the wall of the fruiting body. A variety of appendages may occur on the surface of the chasmothecia. These appendages are thought to act as the hooks of a velcro fastener, attaching the fruiting bodies to the host, particularly to the bark of woody plants, where they overwinter. They can survive winter conditions as dormant mycelia within the buds and other plant tissue of the host. These infected parts of the host can be the source of primary inoculum that can initiate further infection when conditions are right (Misra 2001; Amsalem et al. 2006; Heffer et al. 2006; Te Beest et al. 2008; Saharan et al. 2019; Fig. 1).
Fig. 1 The life cycle of a powdery mildew fungus on roses. Redrawn from Agrios (2005) and Mulbrhan et al. (2016).
Morphological based identification and diversity
Members of Erysiphaceae cause powdery mildew disease on about 10,000 angiosperm species (Takamatsu et al. 2010). The Erysiphaceae are divided into five tribes and two basal genera (Cook et al. 1997). Both tree-parasitic and herb-parasitic species are included in three of the five tribes: Cystotheceae, Erysipheae and Phyllactinieae. Tree-parasitic species usually take basal positions in these tribes and herb-parasitic species have derived positions. The tribe, Golovinomycetea is a group derived from a single ancestor (Mori et al. 2000). The monophyly of the tribe is also supported by the common characteristics, i.e., ectophytic parasitism, polyascal ascomata, and Euoidium asexual morphs, with the latter producing conidia in chains without distinct fibrosin bodies. Of these five lineages, four consists of taxa infectious to dicotyledons. Blumeria graminis, which is infectious to monocotyledon plants, formed an independent lineage. Therefore, Blumeria graminis was accommodated in a monotypic tribe Blumerieae in the new system (Inuma et al. 2007).
The powdery mildew belonging to the tribe Cystotheceae have both herbaceous and woody plants as hosts and consist of three genera, Cystotheca, Podosphaera and Sawadaea, of which Cystotheca and Sawadaea are restricted to a narrow range of host families (Meeboon et al. 2013). Podosphaera consists of two sections, Podosphaera and Sphaerotheca. Section Podosphaera parasitizes woody plants (Takamatsu et al. 2000). The tribe Golovinomyceteae consists of three genera, Golovinomyces, Neoerysiphe, and Arthrocladiella. Arthrocladiella is a monotypic genus consisting of a single species A. mougeottii and has only the host genus Lycium. Neoerysiphe is also a small genus composed of four species and has about 300 herbaceous host species ranging across five plant families including Lamiaceae. Golovinomyces is a large genus comprising 27 species (Braun 1987), and it is widely distributed in the world. The tribe Phyllactinieae comprises the genera Phyllactinia, Leveillula, Pleochaeta and Queirozia which typically have hemi-endophytic (partly external and partly internal mycelia in common (Braun 1987; Liberato 2007; Liberato et al. 2006; Khodaparast et al. 2001; Ramos et al. 2013).
The tribe Erysipheae forms a separate, monophyletic clade, which is characterized by asexual morphs belonging to Oidium subgen. Pseudoidium Jacz (Takamatsu et al. 1999; Mori et al. 2000). This clade comprises Erysiphe and its sections Erysiphe, Microsphaera and Uncinula. Uncinula forestalis differs from the species of Erysiphe sect. Uncinula in having terminal, fasciculate, septate, ascoma appendages and Euoidium-like asexual morph (conidia catenate) and therefore it was placed in Caespitotheca (Takamatsu et al. 2005b). Because of the lack of asexual morphs in Uncinula septata and U. curvispora and multiseptate chasmothecial appendages arising from the upper half the fruiting body, the two species were assigned to Parauncinula (Braun and Takamatsu 2000; Takamatsu et al. 2005a). A unique taxon, Oidium phyllanthi, on Phyllanthus acidus, P. amarus and P. reticulatus produces a germination type designated as Microidium-type and was placed in a new genus Microidium (To-anun et al. 2005). With these new classifications, Erysiphales contains 17 accepted genera, 16 based on the holomorph and one on the asexual morph (Braun and Cook 2012). With the descriptions of several new species, the number of recognized powdery mildew species has increased from 515 (including 435 sexual morphs/holomorphs) in Braun (1987), to about 820 species (including about 685 sexual morphs/holomorphs) (Braun and Takamatsu 2000; Braun et al. 2002; Takamatsu et al. 2005a; b; Liberato et al. 2006; Braun and Cook 2012).
Molecular based identification and diversity
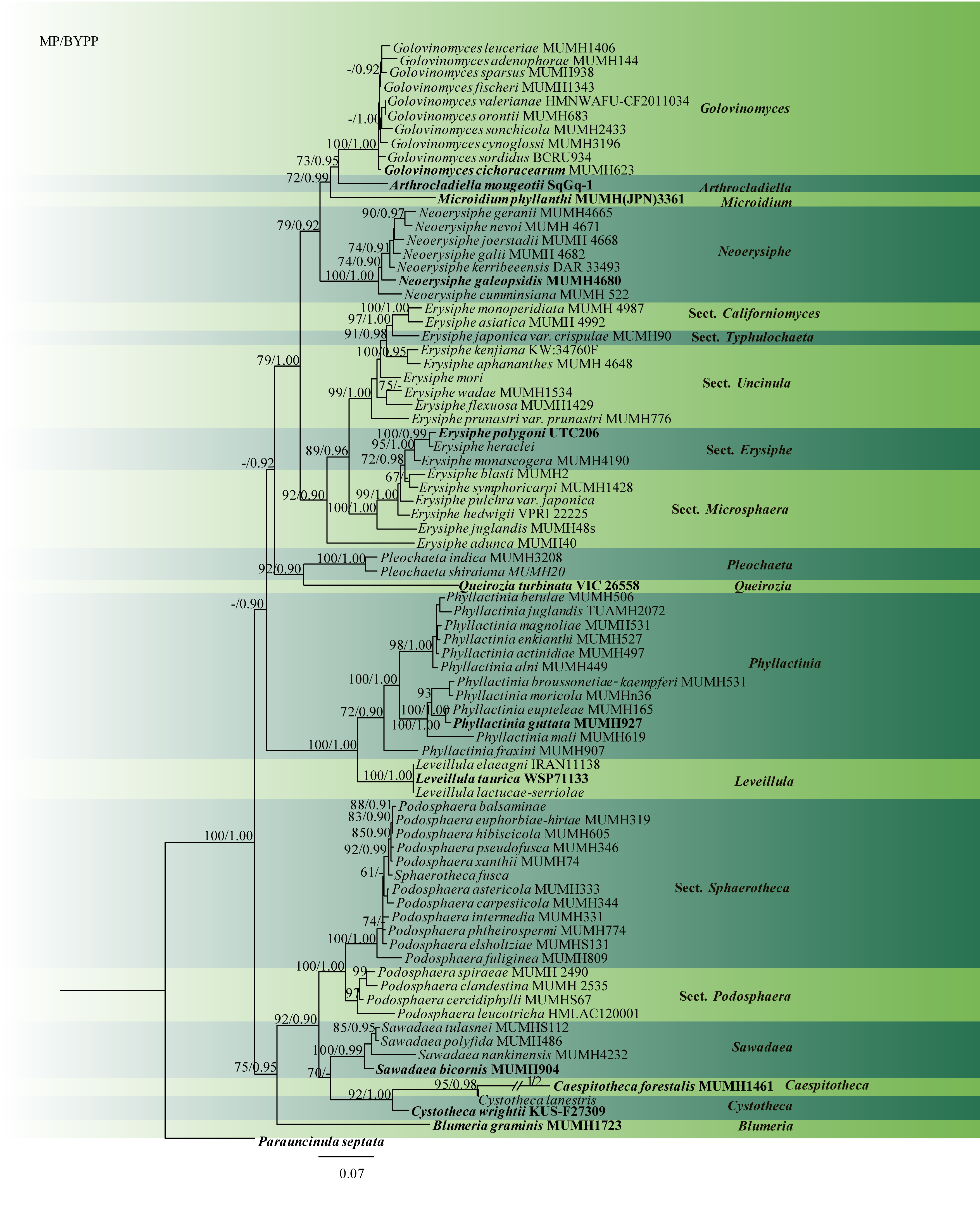
Molecular data have proven useful in reassessing species and clarifying the taxonomic significance of morphology and host data. Only a few of the described species have been reassessed using molecular data (Braun and Cook 2012). Reports began appearing in the 1990s, that used ITS and 18S rDNA sequences to infer phylogenetic relationships of Erysiphales and other major ascomycete groups (Saenz and Taylor 1999; Saenz et al. 1994). Analyses of 18S rDNA, ITS1–5.8S-ITS2, and 28S rDNA sequences led to the opinion that Erysiphales can be placed in Leotiomycetes along with Cyttariales, Helotiales, and Rhytismatales (Wang et al. 2006). Phylogenetic analyses demonstrated that Erysiphaceae formed a distinct monophyletic group (Hirata et al. 2000). Thus, Erysiphaceae is derived from a single ancestral taxon that may have acquired parasitism just once (Mori et al. 2000a; Takamatsu 2004; Wang et al. 2006). Shirouzu et al. (2020) using nrDNA and mcm7 sequence data showed that Phyllactinieae is not monophyletic. However, there is a need to re-assess the tribes in this family to establish them as subfamilies or genera. In this paper, we present a phylogenetic tree with combined ITS and LSU sequences obtained from available type material and voucher specimens (Table 1, Fig. 2). This can be used as a backbone in the identification of powdery mildew species.
Recommended genetic markers (genus level) – ITS, LSU and SSU
Recommended genetic markers (species level) – tub2, chs, tef1
The ITS region of the precursor molecules of rRNA was revealed to form a secondary structure including several stem-loop structures, and some conserved sequences are found in the stem regions (Takamatsu et al. 1998). This makes it possible to design PCR primers that work for a wide range of the powdery mildews. Takamatsu and Kano (2001) designed four new PCR primers that are useful to determine the nucleotide sequences of the rDNA of the powdery mildews. These primers provide stability to work on a wide range of powdery mildews and specificity to eliminate contaminating DNA by PCR. Primer sets PM3/P3, ITS1/PM4, PM5/P3, and ITS1/PM6 were tested with universal primer set ITS1/ITS4 (White et al. 1990) covering all major clades of Erysiphales. Meeboon and Takamatsu (2013a) used LSU, ITS and IGS (Inter generic spacer) sequences to identify two different genetic groups of Erysiphe japonica (= Typhulochaeta japonica), powdery mildew on Quercus species based on the differences in host range. Cho et al. (2014) used ITS and 28S rDNA for the introduction of the powdery mildew species Erysiphe magnoliicola in Erysiphe sect. Microsphaera. Wang et al. (2014) also used ITS differences for phylogenetic analysis of powdery mildew disease on mulberry in Yunnan Province. Meeboon and Takamatsu (2013b) also used the 28S rDNA sequences and a combined alignment of the 28S, ITS, and IGS (Intergeneric spacer) rDNA sequences to construct a phylogeny of Erysiphe sect. Uncinula on Carpinus species and showed the cryptic species Erysiphe paracarpinicola. de Oliveira et al. (2015) used ITS sequences of Erysiphe platani on Platanus × acerifolia in Brazil as new records of taxa. Liyanage et al. (2017) used ITS, SSU and LSU sequences to identify E. quercicola infected rubber trees. Phylogenetic analyses of B. graminis based on the DNA sequences of four DNA regions, i.e. ITS, 28S rDNA, chitin synthase 1, and ß-tubulin were conducted by Inuma et al. (2007) to revealed distinct groups in the B. graminis isolates from a single host genus belonged to a single group.
Table 1 Genera in Erysiphaceae. Type strains are in bold.
| Genera | Species name | Strain no | Host | ITS | LSU |
| Arthrocladiella | Arthrocladiella mougeotii | SqGq-1 | Lycium chinense | JX546296 | AB022379 |
| Blumeria | Blumeria graminis | MUMH1723 | Festuca arundinacea | AB273556 | AB103065 |
| Brasiliomyces | Brasiliomyces malvastri | NA | Malvastrum coromandelianum | NA | NA |
| Caespitotheca | Caespitotheca forestalis | MUMH1461 | Schinopsis balansae | AB193466 | AB193467 |
| Cystotheca | Cystotheca wrightii | KUS-F27309 | Quercus glauca | KF735066 | AB022355 |
| Cystotheca lanestris | NA | Quercus agrifolia | AB000933 | AB022353 | |
| Erysiphe | Erysiphe polygoni | UTC206 | Polygonum arenastrum | AF011307 | NA |
| Erysiphe heraclei | NA | Daucus carota | AB000942 | AB103371 | |
| Erysiphe monascogera | MUMH4190 | Styrax japonica | AB331647 | NA | |
| Erysiphe blasti | MUMH2 | Lindera umbellata | AB015918 | NA | |
| Erysiphe juglandis | MUMH48s | Microsphaera juglandis | AB015928 | NA | |
| Erysiphe hedwigii | VPRI 22225 | NA | AF298539 | NA | |
| Erysiphe pulchra var. japonica | MUMH90 | Cornus controversa | AB000941 | AB022389 | |
| Erysiphe symphoricarpi | MUMH1428 | Symphoricarpos albus | AB078970 | NA | |
| Erysiphe asiatica | MUMH 4992 | Castanopsis diversifolia | AB622218 | JQ220158 | |
| Erysiphe monoperidiata | MUMH 4987 | Castanopsis argyrophylla | AB622213 | JQ220154 | |
| Erysiphe japonica var. crispulae | NA | Quercus cuspidata | AB022416 | AB022415 | |
| D84383 | AB022374 | ||||
| Erysiphe adunca | MUMH40 | NA | |||
| Erysiphe aphananthes | MUMH 4648 | Aphananthe aspera | AB69396 | NA | |
| Erysiphe flexuosa | MUMH1429 | Aesculus hippocastanum | AB091774 | NA | |
| Erysiphe mori | NA | Morus bombycis | AB000946 | AB022418 | |
| Erysiphe kenjiana | KW:34760F | Ulmus minor | AB475118 | AB475109 | |
| Erysiphe prunastri var. prunastri | MUMH776 | Prunus domestica | AB046984 | AB709961 | |
| Erysiphe wadae | MUMH1534 | Fagus crenata | AB091776 | NA | |
| Golovinomyces | Golovinomyces cichoracearum | MUMH623 | Mycelis muralis | AB077661 | NA |
| Golovinomyces adenophorae | MUMH144 | Adenophora triphylla var. japonica | AB077633 | AB077632 | |
| Golovinomyces cynoglossi | MUMH3196 | Cynoglossum asperrimum | AB769454 | AB077683 | |
| Golovinomyces fischeri | MUMH1343 | Senecio doronicum | AB769450 | AB769452 | |
| Golovinomyces leuceriae | MUMH1406 | Leuceria thermarum | AB246765 | NA | |
| Golovinomyces orontii | MUMH683 | Cirsium japonicum | AB769413 | AB077678 | |
| Golovinomyces sonchicola | MUMH2433 | Sonchus arvensis | AB077673 | AB077672 | |
| Golovinomyces sordidus | BCRU394 | Plantago sp. | AB769467 | AB077657 | |
| Golovinomyces sparsus | MUMH938 | Euphorbia collina | AB769461 | NA | |
| Golovinomyces valerianae | HMNWAFU-CF2011034 | Valeriana officinalis | AB769471 | NA | |
| Leveilluta | Leveilluta taurica | WSP71133 | Triglochin maritime | AY912077 | NA |
| Leveillula elaeagni | IRAN11138 | Elaeagnus angustifolia | AB048350 | NA | |
| Leveillula lactucae-serriolae | NA | Hexinia polydichotoma | HQ821500 | HQ821501 | |
| Microidium | Microidium phyllanthi | MUMH(JPN)3361 | Phyllanthus acidus | AB719943 | AB120758 |
| Neoerysiphe | Neoerysiphe cumminsiana | MUMH 522 | Cacalia delphiniifolia | AB329669 | NA |
| Neoerysiphe galeopsidis | MUMH4680 | Chelonopsis moschata | AB498949 | AB022369 | |
| Neoerysiphe galii | MUMH 4682 | Galium aparine | AB498951 | AB103365 | |
| Neoerysiphe geranii | MUMH4665 | Geranium sp. | AB498956 | AB498952 | |
| Neoerysiphe joerstadii | MUMH 4668 | Phagnalon rupestre | AB498976 | NA | |
| Neoerysiphe kerribeeensis | DAR 33493 | Senecio glossanthus | GU356546 | NA | |
| Neoerysiphe nevoi | MUMH 4671 | Scolymus hispanicus | AB498975 | NA | |
| Parauncinula | Parauncinula septata | MUMH585 | Quercus cuspidata var. horikawae | AB183533 | AB022420 |
| Phyllactinia | Phyllactinia guttata | MUMH927 | Corylus sp. | AB080565 | AB080456 |
| Phyllactinia actinidiae | MUMH497 | Actinidia arguta | AB080508 | HQ821501 | |
| Phyllactinia alni | MUMH449 | Alnus japonica | AB080502 | AB080393 | |
| Phyllactinia betulae | MUMH506 | Betula platyphylla var. japonica | AB080507 | AB080398 | |
| Phyllactinia broussonetiaekae-mpferi | MUMH531 | Broussonetia kazinoki | AB080510 | AB080445 | |
| Phyllactinia enkianthi | MUMH527 | Lyonia ovalifolia var. elliptica |
AB080504 | AB080408 | |
| Phyllactinia eupteleae | MUMH165 | Euptelea polyandra | AB080498 | AB080402 | |
| Phyllactinia fraxini | MUMH907 | Syringa vulgaris | AB080543 | AB080453 | |
| Phyllactinia guttata | MUMH927 | Corylus sp. | AB080565 | AB080394 | |
| Phyllactinia juglandis | TUAMH2072 | Juglans mandshurica var. sachalinensis |
AB080531 | AB080422 | |
| Phyllactinia magnoliae | MUMH531 | Magnolia quinquepeta | AB080526 | AB080416 | |
| Phyllactinia mali | MUMH619 | Crataegus sp. | AB080523 | AB080414 | |
| Phyllactinia moricola | MUMHn36 | Morus cathayana | AB080518 | AB080373 | |
| Pleochaeta | Pleochaeta indica | MUMH3208 | Celtis australis | AB243757 | NA |
| Pleochaeta shiraiana | MUMH20 | NA | D84380 | AB022403 | |
| Podosphaera | Podosphaera myrtillina | NA | Vaccinium myrillus | NA | NA |
| Podosphaera leucotricha | HMLAC120001 | Photinia serrulata | JQ999954 | NA | |
| Podosphaera spiraeae | MUMH 2490 | Spiraea cantoniensis | AB525940 | AB022384 | |
| Podosphaera cercidiphylli | MUMHS67 | Cercidiphyllum japonicum | AB026140 | NA | |
| Podosphaera clandestine | MUMH 2535 | Spiraea japonica | AB525941 | AB103367 | |
| Podosphaera astericola | MUMH333 | Aster ageratoides subsp. Ovatus | AB040335 | AB462779 | |
| Podosphaera balsaminae | NA | Impatiens balsamina | FJ625796 | AB462788 | |
| Podosphaera carpesiicola | MUMH344 | Carpesium abrotanoides | AB040350 | NA | |
| Podosphaera elsholtziae | MUMHS131 | Ajuga reptans | AB026142 | AB462794 | |
| Podosphaera euphorbiae-hirtae | MUMH319 | Acalypha australis | AB040306 | AB462770 | |
| Podosphaera fusca | NA | NA | AF154324 | AB103369 | |
| Podosphaera fuliginea | MUMH809 | Verbena spicata | AB046986 | AB462761 | |
| Podosphaera hibiscicola | MUMH605 | Hibiscus mutabilis | AB040308 | NA | |
| Podosphaera intermedia | MUMH331 | Clerodendrum trichotomum | AB026145 | AB462777 | |
| Podosphaera phtheiros-permi | MUMH774 | Melampyrum nemorosum | AB040332 | NA | |
| Podosphaera pseudofusca | MUMH346 | Fatoua villosa | AB040320 | NA | |
| Podosphaera xanthii | MUMH74 | Youngia denticulata | AB040351 | JX512556 | |
| Queirozia | Queirozia turbinata | VIC 26558 | Platycyamus regnellii | AB218773 | NA |
| Sawadaea | Sawadaea bicornis | MUMH904 | Acer pseudoplatanus | AB193380 | AB103370 |
| Sawadaea nankinensis | MUMH4232 | Acer buergerianum | AB353762 | NA | |
| Sawadaea polyfida | MUMH486 | Acer amoenum var. matsumurae | AB193358 | AB193393 | |
| Sawadaea tulasnei | MUMHS112 | Acer mono var. marmoratum | AB193388 | AB193400 | |
| Takamatsuella | Takamatsuella circinata | NA | Stachys distans | NA | NA |
Fig 2 Phylogram generated from parsimony analysis based on combined ITS and LSU sequenced data Erysiphaceae. Maximum parsimony bootstrap support values greater than 60 % and BYPP greater than 0.90 are indicated above the nodes. The type specimens (ex-epitypes) are in bold. The tree is rooted with Parauncinula septata.

No Comments