20 Sep Neofusicoccum
Neofusicoccum Crous, Slippers & A.J.L. Phillips, Stud. Mycol. 55: 247 (2006)
Background
When Crous et al. (2006) split Botryosphaeria into ten distinct genera they introduced Neofusicoccum for species morphologically similar to, but phylogenetically distinct from Botryosphaeria sensu lato. Despite the similar morphology, Crous et al. (2006) considered that the Dichomera-like syn-asexual morph seen in some Neofusicoccum species distinguishes it from Botryosphaeria. However, the Dichomera-like syn-asexual morph has not been found in all species of Neofusicoccum and it is not produced consistently by all isolates of those species that are known to possess this state. Phillips et al. (2013) suggested that paraphyses, which have never been reported in conidiomata of Neofusicoccum but are known in some species of Botryosphaeria, might be a suitable character to separate the two genera. However, the similarity of paraphyses to developing conidiogenous cells makes this feature difficult to apply. Furthermore, paraphyses have not been reported in all Botryosphaeria species.
Classification – Dothideomycetes, incertae sedis, Botryosphaeriales, Botryosphaeriaceae
Type species – Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, in Crous et al., Stud. Mycol. 55: 248 (2006)
Distribution – Worldwide
Disease Symptoms – Dieback, Canker, Fruit rot
Hosts – Plurivorous on woody hosts
Morphological based identification and diversity
Currently, 43 species are known in Neofusicoccum. Cultures and DNA sequence data are available for all the known species. Although Yang et al. (2017) and Li et al. (2018) included isolates of N. terminaliae in their phylogenetic analyses, no record of this species name could be found in MycoBank or Index Fungorum, but sequences are available in GenBank and a CBS culture collection number was quoted by Li et al. (2018). Since sequence data and culture are available we provisionally include N. terminaliae as a species in Neofusicoccum. Morphologically the species are differentiated based on conidial dimensions, colouration and septation in aged conidia and pigment production in culture. Phillips et al. (2013) attempted to construct a key for identification of 22 species, but in reality plasticity of characters and overlapping of conidial dimensions rendered this attempt unreliably. Considering that a further 21 species have been introduced in Neofusicoccum since then the only reliable way to identify species is with DNA sequence data.
Species cannot be identified reliably on the basis of morphological characters alone due to plasticity and overlapping of conidial dimensions.
Molecular based identification and diversity
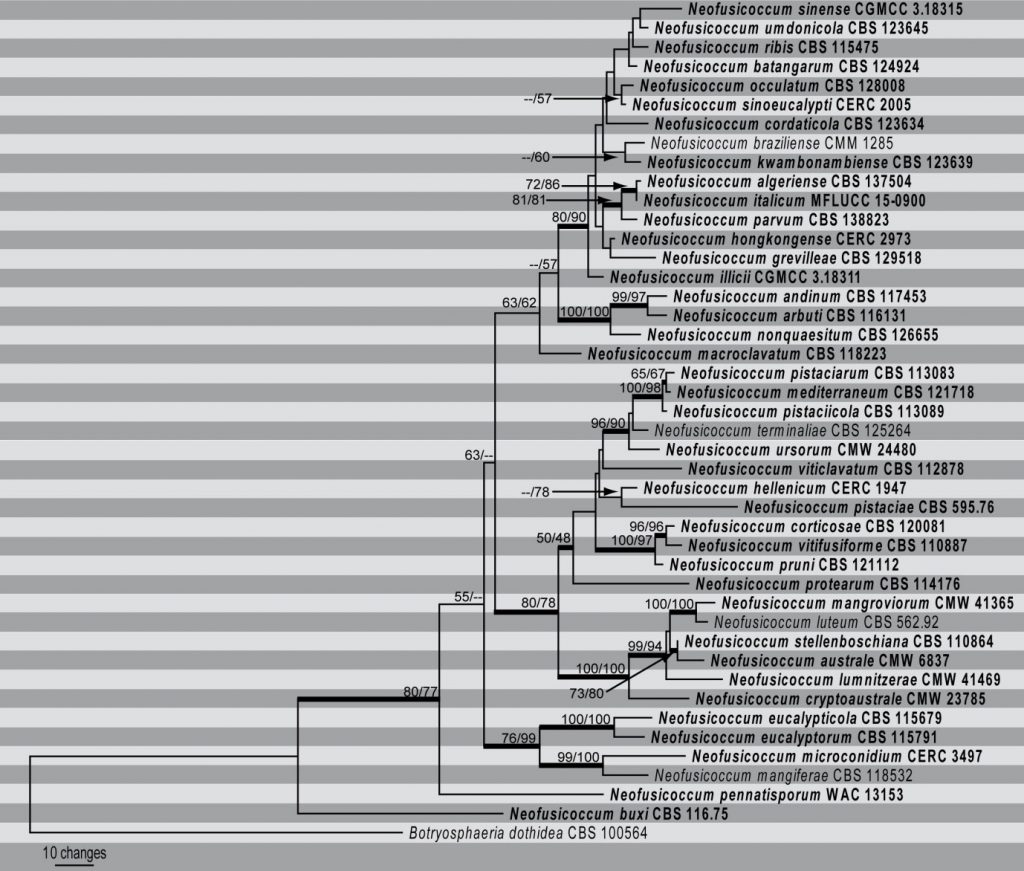
Species in Neofusicoccum can be distinguished with a combination of ITS and partial TEF1- α sequences. In this way, Phillips et al. (2013) distinguished 22 species while Dissanayake et al. (2016) distinguished 29 species. However, resolution of species within some complexes is not always clearly defined and for that reason, Hyde et al. (2014) recommended the use of ITS, TEF1- α and TUB2 sequence data to separate the 22 species they included in Neofusicoccum. More recently, Marin-Felix et al. (2017) used a combination of ITS, TEF1- α, TUB2 and RPB2 sequence data to resolve 34 species. Yang et al. (2017) used the same combination of loci to differentiate 31 named species and a further nine lineages that they declined to name. Li et al. (2018) also used a combination of ITS, TEF1- α, TUB2 and RPB2 sequence data when they introduced a further two species collected from China. Considering the recent trends we use the same combination of ITS, TEF1- α, TUB2 and RPB2 sequence data to separate 43 species in Neofusicoccum (Fig).
While most of the species are clearly accommodated within Neofusicoccum, N. pennatisporum and N. buxi are phylogenetically divergent and morphologically atypical of the genus. The extremely long conidia of N. pennatisporum (40–50 µm long) that can be up to 5-septate and ascospores with apical protrusions (Taylor et al. 2009) are unlike any other known species in Neofusicoccum. Conidia of N. buxi (Yang et al. 2017) are atypically shaped (sub-cylindrical) and unusually large (30–38×7–8 µm) for a species in Neofusioccum. Together with the divergent phylogeny, these are sufficient reasons to question the inclusion of these two species in Neofusicoccum.
Recommended genetic markers (genus level) – SSU, LSU
Recommended genetic markers (species level) – ITS, TEF1- α, TUB2, RPB2
Even though it is possible to distinguish all species with a combination of ITS and TEF1- α, some species complexes are resolved more clearly with the addition of TUB2 and RPB2 sequence data.
Accepted number of species: There are 41 valid species epithets in Index Fungorum (August 2018) and 41 in MycoBank (August 2018) under this genus. However, some names have since been validated and we currently accept 43 species names in Neofusicoccum.
References: Phillips et al. 2013 (morphology, phylogeny, hosts), Dissanayake et al. 2016 (phylogeny, hosts, species numbers).
Table. Neofusicoccum. Details of the isolates used in the phylogenetic analyses. Ex-type (ex-epitype) strains are in bold and marked with an * and voucher strains are in bold
| Species | Isolate | ITS | TEF1- α | tub-2 | RPB2 |
| N. algeriense | CBS 137504* | KJ657702 | KJ657715 | KX505915 | N/A |
| N. andinum | CBS 117453* | AY693976 | AY693977 | KX464923 | KX464002 |
| N. arbuti | CBS 116131* | AY819720 | KF531792 | KF531793 | KX464003 |
| N. austral | CMW 6837* | AY339262 | AY339270 | AY339254 | EU339573 |
| N. batangarum | CBS 124924* | FJ900607 | FJ900653 | FJ900634 | FJ900615 |
| N. braziliense | CMM 1285 | JX513628 | JX513608 | KC794030 | N/A |
| N. buxi | CBS 116.75* | KX464165 | KX464678 | N/A | KX464010 |
| N. cordaticola | CBS 123634* | EU821898 | EU821868 | EU821838 | EU821928 |
| N. corticosae | CBS 120081* | DQ923533 | KX464682 | KX464958 | KX464013 |
| N. cryptoaustrale | CMW 23785* | FJ752742 | FJ752713 | FJ752756 | KX464014 |
| N. eucalypticola | CBS 115679* | AY615141 | AY615133 | AY615125 | N/A |
| N. eucalyptorum | CBS 115791* | AF283686 | AY236891 | AY236920 | N/A |
| N. grevilleae | CBS 129518* | JF951137 | N/A | N/A | N/A |
| N. hellenicum | CERC 1947* | KP217053 | KP217061 | KP217069 | N/A |
| N. hongkongensis | CERC 2973* | KX278052 | KX278157 | KX278261 | KX278283 |
| N. ilicii | CGMCC 3.18311* | KY350150 | KY817756 | KY350156 | N/A |
| N. italicum | MFLUCC 15-0900* | KY856755 | KY856754 | N/A | N/A |
| N. kwambonambiense | CBS 123639* | EU821900 | EU821870 | EU821840 | EU821930 |
| N. lumnitzerae | CMW 41469* | KP860881 | KP860724 | KP860801 | KU587925 |
| N. luteum | CBS 562.92 | KX464170 | KX464690 | KX464968 | KX464020 |
| N. macroclavatum | CBS 118223* | DQ093196 | DQ093217 | DQ093206 | KX464022 |
| N. mangiferae | CBS 118531* | AY615185 | DQ093221 | AY615173 | KX464023 |
| N. mangroviorum | CMW 41365* | KP860859 | KP860702 | KP860779 | KU587905 |
| N. mediterraneum | CBS 121718* | GU251176 | GU251308 | N/A | KX464024 |
| N. microconidium | CERC 3497* | KX278053 | KX278158 | KX278262 | MF410203 |
| N. nonquaesitum | CBS 126655* | GU251163 | GU251295 | GU251823 | KX464025 |
| N. occulatum | CBS 128008* | EU301030 | EU339509 | EU339472 | EU339558 |
| N. parvum | CBS 138823* | AY236943 | AY236888 | AY236917 | EU821963 |
| N. pennatisporum | MUCC 510* | EF591925 | EF591976 | EF591959 | N/A |
| N. pistaciae | CBS 595.76* | KX464163 | KX464676 | KX464953 | KX464008 |
| N. pistaciarum | CBS 113083* | KX464186 | KX464712 | KX464998 | KX464027 |
| N. pistaciicola | CBS 113089* | KX464199 | KX464727 | KX465014 | KX464033 |
| N. protearum | CBS 114176* | AF452539 | KX464720 | KX465006 | KX464029 |
| N. pruni | CBS 121112* | EF445349 | EF445391 | KX465016 | KX464034 |
| N. ribis | CBS 115475* | AY236935 | AY236877 | AY236906 | EU339554 |
| N. sinense | CGMCC 3.18315* | KY350148 | KY817755 | KY350154 | N/A |
| N. sinoeucalypti | CERC 2005* | KX278061 | KX278166 | KX278270 | KX278290 |
| N. stellenboschiana | CBS 110864* | AY343407 | AY343348 | KX465047 | KX464042 |
| N. terminaliae | CBS 125264 | GQ471802 | GQ471780 | KX465053 | KX464046 |
| N. umdonicola | CBS 123645* | EU821904 | EU821874 | EU821844 | EU821934 |
| N. ursorum | CMW 24480* | FJ752746 | FJ752709 | KX465056 | KX464047 |
| N. viticlavatum | CBS 112878* | AY343381 | AY343342 | KX465058 | KX464048 |
| N. vitifusiforme | CBS 110887* | AY343383 | AY343343 | KX465061 | KX464049 |
| Botryosphaeria dothidea | CBS 100564 | KX464085 | KX464555 | KX464781 | KX463951 |
Fig. First of 1000 most parsimonious trees resulting from analysis of combined ITS, TEF1-α, tub2 and RPB2 sequence data. Forty-three strains were included in the analyses, which comprise 1829 characters including gaps. The tree was rooted with Botryosphaeria dothidea. (CBS 100564). The topology of the MP tree was similar to that of the ML and BI trees. The maximum parsimony dataset consisted of 1829 characters of which 1335 were constant, 241 variable characters were parsimony uninformative. Analysis of the remaining 253 parsimony-informative characters resulted in 1000 equally most parsimonious trees with a length of 873 steps and CI = 0.674, RI = 0.762, HI = 0.326. The best scoring RAxML tree had a final likelihood value of -7311.594985. The matrix had 583 distinct alignment patterns, with 14.54% of undetermined characters or gaps. Estimated base frequencies were as follows; A = 0.214778, C = 0.295225, G = 0.272849, T = 0.217148; substitution rates AC = 1.184543, AG = 5.593626, AT = 1.053227, CG = 1.490317, CT = 11.314839, GT = 1.000000; gamma distribution shape parameter α = 0.472397. Bootstrap values for MP followed by ML are given at the nodes Thickened lines represent Bayesian posterior probability scores >0.95. Ex-type and ex-epitype isolates are in bold

No Comments