27 Oct Phellinus
Phellinus Quél., Enchir. fung. (Paris): 172 (1886)
Background
Phellinus was introduced by Quélet (1886) with P. igniarius (≡ Boletus igniarius) as its type species (Murrill 1903) and is placed in Hymenochaetaceae (He et al. 2019). Traditionally, most poroid Hymenochaetaceae were placed in Phellinus, which has been characterized by a dimitic hyphal system and perennial habit of the basidiomata (Gilbertson 1979; Larsen and Cobb-Poulle 1990; Ryvarden and Gilbertson 1994; Núñez and Ryvarden 2000). However, phylogenetic studies revealed that the morphologically defined Phellinus sensu lato had polyphyletic origins within the Hymenochaetoid clade, and most species previously classified as Phellinus are now members of various segregate genera (e.g. Wagner and Fischer 2001, 2002; Jeong et al. 2005; Dai 2010; Rajchenberg et al. 2015; Drechsler-Santos et al. 2016). According to the most narrowly defined generic concept, Phellinus sensu stricto is limited to the P. igniarius species complex (Fischer and Binder 2004), which includes species causing a delignifying trunk rot mostly on various deciduous trees in temperate areas (Brazee 2015; Zhou et al. 2016). Based on a wider generic concept, several morphologically similar species described from East Asia, Africa or America are considered as part of Phellinus sensu stricto (Decock et al. 2006; Yombiyeni et al. 2011; Cui and Decock 2013; Bian et al. 2016; Campos-Santana et al. 2016; Vlasák and Vlasák 2017; Salvador-Montoya et al. 2018; Zhu et al. 2018). In this study, we follow the broader concept of classification of Phellinus sensu stricto, pending further studies.
Classification – Agaricomycotina, Basidiomycota, Agaricomycetes, Incertae sedis, Hymenochaetales, Hymenochaetaceae
Type species – Phellinus igniarius (L.) Quél., Enchir. fung. (Paris): 177 (1886)
Distribution – If the wider generic concept of Phellinus were accepted it would be a globally distributed genus, with certain species found in East Asia, Europe, North America (Dai 2010; Cui and Decock 2013; Brazee 2015; Zhou et al. 2016; Vlasák and Vlasák 2017; Zhu et al. 2018), Central- and South America (Decock et al. 2006; Campos-Santana et al. 2016; Salvador-Montoya 2018) and Africa (Yombiyeni et al. 2011; Cloete et al. 2016). However, the members of the P. igniarius species complex are known only from the Northern Hemisphere (Brazee 2015; Zhou et al. 2016).
Disease symptoms – Members of Phellinus produce white rot, decaying polysaccharides and delignifying the substrata (Niemelä 1974, 1977; Ryvarden and Gilbertson 1994; Wagner and Fischer 2002; Decock et al. 2006; Cui and Decock 2013; Brazee 2015; Cloete et al. 2016, de Campos-Santana et al. 2016). The rot could be localized in the trunk as a column of decay (Brazee 2015), in both fallen and in standing dead trunks (Niemelä 1977; Campos-Santana et al. 2016). Branches of living trees (Niemelä 1974; Decock et al. 2006), dead, fallen, corticated branches and logs (Niemelä 1972) and dead stumps (Niemelä 1972; Decock et al. 2006; Campos-Santana et al. 2016) are colonized and decayed. The fungus penetrates the heartwood, causing heartrot (Niemelä 1974; Larsson et al. 2006), sometimes extending into the sapwood (Niemelä 1977; Larsson et al. 2006). Decay characteristics (i.e. colour, fragility and fragmentation) vary between species (Niemelä 1972, 1974, 1977; Yombiyeni et al. 2011; Luna et al. 2012; Campos-Santana et al. 2016). Pathogenic species, such as P. tremulae or P. resupinatus are usually associated with other basidiomycete species, pathogenic bacteria and basal fungi (Kallio 1972; Cloete et al. 2016). Phellinus tremulae is a common and harmful pathogen of aspen (Populus species), penetrating the heartwood along dead branches (Niemelä 1974), but is also capable of spreading through the sapwood (Larsson et al. 2006), forming conks around the decayed tissues (Jones 1998; Fig 25). Phellinus resupinatus is also a factor of Esca disease, causing white rot and decline of the cordons in vineyards (Cloete et al. 2016), besides other symptoms caused by this disease (Jayawardena et al. 2019a).
Hosts – Most species in the P. igniarius species complex are specialized to a single or few angiosperm genera (Fischer and Binder 1995; Zhou et al. 2016), and only P. piceicola has been reported from gymnosperms (Cui and Dai 2012). Species of the P. igniarius species complex have been recorded from various host genera, such as Acer, Alnus, Arctostaphylos, Betula, Carpinus, Fagus, Fraxinus, Laburnum, Picea, Populus, Prunus, Salix, Sorbus and Tilia (Tomšovský et al. 2010; Brazee 2015; Zhou et al. 2016). The members of other Phellinus sensu stricto lineages are known from several additional angiosperm genera, such as Artemisia, Astronium, Caesalpinia, Carya, Castanopsis, Dimorphandra, Minquartia, Morus, Sacaglottis, Schinopsis, Quercus and Vitis (Lombard and Larsen 1985; Decock et al. 2006; Yombiyeni et al. 2011; Cui and Decock 2013; de Campos-Santana et al. 2016; Vlasák and Vlasák 2017; Salvador-Montoya et al. 2018).
Morphological based identification and diversity
Phellinus in a wider sense is morphologically heterogenous. The main features of the P. igniarius species complex are the crusted pileal surface (except resupinate species), the hymenial setae arising from the subhymenium (except specimens of “P. pseudoigniarius”, see Dai and Yang 2008; Zhou et al. 2016), and the colourless, inamyloid, indextrinoid and weakly cyanophilous basidiospores (Wagner and Fischer 2001; Dai 2010; Zhou et al. 2016). In many cases, the species separation in the complex is difficult when solely based on morphological characters (Sell 2008). Host preference is also widely used for delimiting species (Tomšovský et al. 2010).
Similar to members of the P. igniarius species complex, other Phellinus species also have perennial basidiomata, but differ in having distinctive macroscopical features (e.g. size and shape of pores, rimose surface, cracked basidiocarps, absence of pileus crust, see Dai et al. 2008; Bian et al. 2016; Cloete et al. 2016; Vlasák and Vlasák 2017) or microscopic characteristics (e.g. hyphal structure, the shape of setae, basidiospore reaction in chemical solutions). For example, P. bicuspidatus is unique in having a monomitic hyphal system with short bicuspid setae (Lombard and Larsen 1985; Cloete et al. 2016). Members of the P. ellipsoideus group are well-characterised by their weakly dextrinoid basidiospores and hooked hymenial setae (Zhu et al. 2018).
There are several “Phellinus” species which have been described solely on morphological features. The status of these species should be critically re-evaluated based on molecular evidence. Amongst these, certain species (e.g. P. deuteroprunicola, P. eugeniae, P. formosanus, P. livescens, P. prunicola, P. setulosus, P. tenuiculus, P. wahlbergii) may belong to Phellinus sensu stricto (Gilbertson 1979; Chang 1995; Chang and Chou 1999, 2000; Robledo et al. 2003; Wang et al. 2011; Rajchenberg et al. 2015; Campos-Santana et al. 2016), but further studies are required to confirm their placements.
Fig. 1 Phellinus igniarius. a causing white-rot decay on willow b–d basidiomes on living willow e–f hymenial setae g tramal skeletal hyphae h basidiospores, Scale bars: e–h = 10 µm
Molecular based identification and diversity
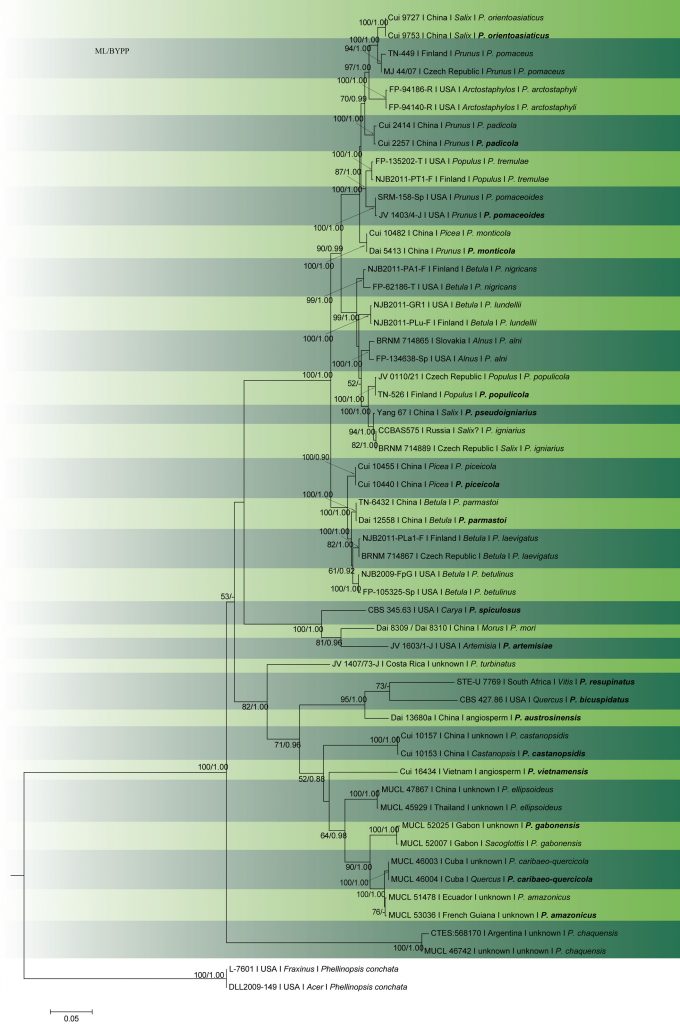
In early molecular studies, the restriction fragment length polymorphism (RFLP) data of enzymatically amplified rDNA was used by Fischer (1995) to study the taxonomy of P. igniarius and its closest relatives in Europe. Later, single nuclear genes (ITS, Fischer and Binder 2004), or combined datasets (ITS-tef1, Tomšovský et al. 2010; Zhou et al. 2016) were used to investigate the species boundaries and phylogenetic relationships within the P. igniarius species complex. Phylogenetic analyses by Brazee (2015) used ITS, LSU, tef1 and rpb2, with isolates representing 13 species-level lineages in the complex. Zhou et al. (2016) distinguished 15 species, five of which are described as new from China and the USA. Based on our multigene analysis (Fig. 26), 16 species can be found in the P. igniarius species complex, distributed throughout the Northern Hemisphere. Amongst these, ten species are known from eastern Asia, eight from Europe and seven from North America.
Phellinus caribaeo-quercicola was the first species described from the “P. ellipsoideus group” based on molecular evidence (Decock et al. 2006). The nLSU-based phylogenetic analysis of Decock et al. (2006), have shown that P. caribaeo-quercicola grouped close to the P. igniarius species complex and some other Phellinus species (viz. P. bicuspidatus, P. chaquensis and P. spiculosus). Later molecular taxonomic studies used combined datasets of various nuclear markers. The combined analyses of nITS, nLSU, tef1 and rpb2 have confirmed the phylogenetic position of P. caribaeo-quercicola and five morphologically similar species have been accepted in Phellinus sensu stricto (Yombiyeni et al. 2011; Cui and Decock 2013; Campos-Santana et al. 2016; Zhu et al. 2018). Currently, this later group consists of six species and mostly has tropical or subtropical distributions (Zhu et al. 2018).
Recommended genetic marker (genus level) – LSU
Recommended genetic markers (species level) – ITS, tef1, rpb2
Accepted number of species – There are 479 epithets listed in Index Fungorum (2020. However, most of the species belong to other poroid Hymenochaetaceae genera, such as Fomitiporia, Fomitiporella, Fulvifomes, Fuscoporia, Nothophellinus, Phellinidium, Phellinopsis, Phellinotus, Phellopilus, Phylloporia, Porodaedalea, Sanghuangporus and Tropicoporus (Wagner and Fischer 2001, 2002; Niemelä et al. 2001; Dai 2010; Drechsler-Santos et al. 2016; Rajchenberg et al. 2015; Zhou et al. 2016). Based on molecular data, 30 species are accepted in Phellinus sensu stricto, from among 16 species in the P. igniarius species complex (Table 1; Fig. 2).
References – Tomšovský et al. (2010) (phylogeny, P. igniarius species complex, Europe), Brazee (2015) (phylogeny, P. igniarius species complex, North America), Zhou et al. (2016) (phylogeny, P. igniarius species complex), Zhu et al. (2018) (phylogeny, P. ellipsoideus group)
Table 1 DNA barcodes available for Phellinus. Ex-type/ex-epitype/ex-neotype/ex-lectotype strains are in bold and marked with an asterisk (*). Voucher strains are also in bold.
| Species | Strain | ITS | LSU | tef1 | rpb2 |
| Phellinus alni | FP-134638-Sp | KU139167 | KU139213 | KU139330 | KU139280 |
| P. alni | BRNM 714865 | GQ383730 | – | GQ383840 | – |
| P. amazonicus | MUCL 53036* | KU499940 | KU376305 | KU936772 | – |
| P. amazonicus | MUCL 51478 | KU499929 | KU376294 | KU936769 | – |
| P. arctostaphyli | FP-94186-R | KU139145 | KU139252 | KU139350 | KU139266 |
| P. arctostaphyli | FP-94140-R | KU139143 | KU139250 | KU139348 | KU139264 |
| P. artemisiae | JV 1603/1-J* | KY230518 | KY230518 | – | – |
| P. austrosinensis | Dai 13680a* | – | KP027474 | – | – |
| P. betulinus | NJB2009-FpG | KU139153 | KU139248 | KU139368 | KU139311 |
| P. betulinus | FP-105325-Sp | KU139154 | KU139239 | KU139369 | KU139312 |
| P. bicuspidatus | CBS 427.86* | MH861982 | MH873674 | – | – |
| P. caribaeo-quercicola | MUCL 46004* | HM635698 | DQ127280 | HM635726 | – |
| P. caribaeo-quercicola | MUCL 46003 | HM635697 | DQ127279 | HM635725 | – |
| P. castanopsidis | CUI 10153* | JQ837944 | JQ837956 | KU936783 | – |
| P. castanopsidis | CUI 10157 | JQ837945 | JQ837957 | KU936784 | – |
| P.chaquensis | MUCL 46742 | – | DQ122396 | – | – |
| P.chaquensis | CTES568170 | MG242440 | MG242445 | – | – |
| P. ellipsoideus | MUCL 47867 | KU954545 | KU954540 | KU936786 | – |
| P. ellipsoideus | MUCL 45929 | KU954544 | DQ127283 | KU936785 | – |
| P. gabonensis | MUCL 52025* | HM635708 | HM635690 | HM635731 | – |
| P. gabonensis | MUCL 52007 | HM635718 | HM635685 | HM635729 | – |
| P. igniarius | BRNM 714889 | GQ383709 | – | GQ383791 | – |
| P. igniarius1 | *Yang 67 | JQ828880 | – | KR013111 | – |
| P. igniarius | CCBAS575 | LN714586 | – | – | LN714693 |
| P. laevigatus | NJB2011-PLa1-F | KU139148 | KU139241 | KU139372 | KU139305 |
| P. laevigatus | BRNM 714867 | GQ383778 | – | GQ383856 | – |
| P. lundellii | NJB2011-PLu-F | KU139185 | KU139234 | KU139337 | KU139300 |
| P. lundellii | NJB2011-GR1 | KU139182 | KU139232 | KU139336 | KU139301 |
| P. monticola | Dai 5413* | JQ828889 | – | KR013087 | – |
| P. monticola | Cui 10482 | JQ828888 | – | KR013086 | – |
| P. mori | Dai 8309/Dai 8310 | FJ627259 | HQ328535 | – | – |
| P. nigricans | NJB2011-PA1-F | KU139169 | KU139222 | KU139340 | KU139297 |
| P. nigricans | FP-62186-T | KU139176 | KU139227 | KU139343 | KU139290 |
| P. orientoasiaticus | Cui 9753* | JQ828926 | – | KR013079 | – |
| P. orientoasiaticus | Cui 9727 | JQ828921 | – | KR013076 | – |
| P. padicola | Cui 2257* | JQ828905 | – | KR013073 | – |
| P. padicola | Cui 2414 | JQ828906 | – | – | – |
| P. parmastoi | Dai 12558* | JQ828900 | – | KR013089 | – |
| P. parmastoi | TN-6432 | KU139158 | KU139245 | KU139376 | – |
| P. piceicola | Cui 10440* | JQ828908 | – | – | – |
| P. piceicola | Cui 10455 | JQ828910 | – | KR013085 | – |
| P. pomaceoides | JV 1403/4-J* | KR013069 | – | KR013107 | – |
| P. pomaceoides2 | SRM-158-Sp | KU139140 | KU139210 | KU139353 | KU139267 |
| P. pomaceus | TN-449 | KU139142 | KU139254 | KU139352 | KU139263 |
| P. pomaceus | MJ 44/07 | GQ383783 | – | GQ383858 | – |
| P. populicola | TN-526* | KU139179 | KU139231 | KU139333 | KU139303 |
| P. populicola | JV 0110/21 | KR013062 | – | KR013093 | – |
| P. resupinatus | STE-U 7769* | KM523246 | KM523251 | – | – |
| P. spiculosus | CBS 345.63* | MH858307 | MH869918 | – | – |
| P. tremulae | NJB2011-PT1-F | KU139132 | KU139201 | KU139357 | KU139276 |
| P. tremulae | FP-135202-T | KU139134 | KU139207 | KU139359 | KU139271 |
| P. turbinatus | JV 1407/73-J | KT156687 | – | – | – |
| P. vietnamensis | Cui 16434* | – | MG867716 | MG867722 | MG867719 |
1as P. pseudoigniarius; 2as PhellinusNA2
Fig. 2 Phylogram generated from RAxML analysis based on combined ITS, LSU, Tef1-α and rpb2 sequence data of Phellinus species. Related sequences were obtained from GenBank. Fifty-five strains are included in the analyses, which comprised 3170 characters including gaps. The tree was rooted with Phellinopsis conchata (DLL2009-149 and L-7601). Tree topology of the ML analysis was similar to the Bayesian analysis.ML bootstrap values ˃50% and BYPP ˃0.80 are shown respectively near the nodes.


No Comments