27 Oct Mucor
Mucor Fresen., Beitr. Mykol. 1: 7 (1850)
Background
Mucor belongs to the order Mucorales, which is among one of the most studied groups of early diverging lineages of fungi. The genus has the largest number of species within the order and half of the sequences submitted to GenBank for Mucorales are of Mucor (Hoffman et al. 2013; Spatafora et al. 2016; Hyde et al. 2014; Nguyen and Lee 2018). Mucor belongs to the phylum Mucoromycota, subphylum Mucoromycotina, class Mucoromycetes, order Mucorales and family Mucoraceae (Wijayawardene et al. 2018, 2020). It was described by Fresenius in 1850 and the type species is Mucor mucedo. Recent molecular studies of mucoralean species have indicated that Mucor is polyphyletic (Nguyen et al. 2017). However, even with definite results showing the polyphyly of Mucor, few clear lineages within Mucor are recognized. Some of these lineages share innate characteristics, such as sporangium size and branching of tall sporangiophores and the morphology is still widely used in current taxonomy (Walther et al. 2013). Analysis of internal transcribed spacer (ITS) and large subunit (LSU) rDNA sequence data of several mucoralean species, showed that some Mucor species with curved sporangiophores grouped with species of Backusella and hence was transferred to Backusella (Walter et al. 2013; Nguyen et al. 2017). Mucor species are commonly isolated from soil, dung, insect, and fruits (Benny 2008). Some species are of biotechnological importance such as biofuel, enzyme, terpernoid production and biotransformation while other species cause mucoromycosis in immunosuppressed humans (Nguyen et al. 2017; Steve et al. 2018; Morin-Sardin 2017). Comparative analyses of five Mucor species based on their lifestyles (M. fuscus and M. lanceolatus (used for cheese production), M. circinelloides and M. racemosus (opportunistic pathogens) and M. endophyticus (an endophyte)) revealed the core transcriptome comprising 5566 orthogroups included genes potentially involved in secondary metabolism. Due to the wide taxonomic range investigated, the five transcriptomes also displayed specificities that can be linked to the different lifestyles, such as differences in the composition of transcripts identified as virulence factors or carbohydrate transporters. Research on this genus has changed its course to identify the link between genetic and biological data, especially in terms of lifestyle and adaptations to a given habitat (Lebreton et al. 2019).
Classification – Zygomycota, Mucoromycotina, Mucoromycetes, Mucorales, Mucorineae, Mucoraceae
Type species – Mucor mucedo Fresen.
Distribution– Worldwide
Disease symptoms –Mucor rot and soft rot
Mucor species especially M. fragilis, M. irregularis, M. piriformis and M. racemosus often cause postharvest diseases such as Mucor rot and soft rot. The initial symptoms of Mucor rot are similar to plant diseases caused by green mold, blue mold, and sour mold. The infected tissue becomes soft and watery. The lesions turn light to dark brown and as the infection progresses, white or shiny grey sporangiophores form at the lesions. Fungal growth spreads across the whole host and masses of sporangiophores bearing black to pale brown sporangia are observed. Decaying fruits become “juicy” within which are abundant spores of the fungus (Li et al. 2014; Saito et al. 2016). Ito et al. (1979) found that three species of fruit flies namely Certitis capitata, Dacus cucurbitae and D. dorsalis, can transmit Mucor rot in guava.
Soft rot caused by Mucor racemosus results in water-soaked appearance followed by a softening of the infected part. When the disease progresses growth of white mycelium and brownish to grey sporangia can be observed. Finally, the infected tissue is broken down and disintegrates in a watery rot (Kwon and Hong 2005; López et al. 2016).
Hosts– Wide host range including, Actinidia deliciosa, Citrus reticulata, Dioscorea species, Fragaria × ananassa, Mangifera indica, Manihot esculenta, Prunus species, Psidium guajava, Solanum melongena, Solanum lycopersicum and Vitis species (Farr and Rossman 2020).
Pathogen biology, disease cycle and epidemiology
The pathogen reproduces asexually. Mucor rot often develops by infecting punctured wounds and cracks on the surface of the fruit, stem end or calyx of the host. In the early stages of the infection, the fruit becomes soft and appears water-soaked. The lesions formed are quasicircular or irregular, light to dark brown and the sporangiophores protrude through the wounds (Kwon and Hong 2005; Saito et al. 2016; Michailides and Spotts 1990). As the infection advances, the infected part disintegrates into a watery rot and the infection spreads and extends to all extremities of the fruit or even the surface of the container. The infected part is covered with a large mass of mycelium with erect sporangiophores and sporangia (Saito et al. 2016; Michalltdes and Spotts 1990). When tested, rotten apple and pear by some Mucor species release an alcoholic odour while Mucor rot in peaches and nectarines caused by M. piriformis emits a pleasant aromatic odour. At an advanced stage, Mucor rot can be distinguished from other rots caused by Rhizopus or Gilbertella. Differences are observed in the mycelial character, growth, sporangiophores and sporangia. For Mucor rot, erect, white or yellowish sporangiophore with grey to black sporangia is observed which covers the decay lesion densely. However, for Rhizopus rot, the mycelia are interwoven with stolons with dark sporangiophores and black sporangia. The sporangial wall eventually dries and falls apart while in Mucor rot, the sporangia absorb water from the sporangial wall which dissolves (Michalltdes and Spotts 1990).
Morphology- based identification and diversity
Mucor is characterized by fast-growing colonies. The sporangiophores are simple or branched without basal rhizoids. However, under some conditions, they form rhizoids. These species normally form globose sporangia, containing the columella and spores. The sporangium is non-apophysate with pigmented and ornamented zygosporangial walls. Arthrospores, chlamydospores, and zygospores may be produced by some species. The zygospores lack appendaged suspenders and broad aseptate or sparsely septate hyphae are commonly found in Mucor species (Nguyen et al. 2016). When spores from sporangia are released, a remaining collarette is observed. The sporangiospores are round or slightly elongated (Larone 1995; Sutton et al. 1998; de Hoog et al. 2000). With 76 accepted species, the genus is the largest and most studied group in Mucorales (Walther et al. 2019).
Molecular identification and diversity
The present taxonomy of Mucor is mostly based on morphological characters and interfertility tests. The genus was previously diagnosed using biological species recognition and morphological species recognition (Schipper 1973; Hermet et al. 2012). However, identification often fails with only morphology hence phylogenetic species recognition has been used to resolve species (Taylor et al. 2000). The use of multi-gene (ITS, tef1 and act) phylogenetic analysis showed that Mucor is not monophyletic (Nguyen et al. 2017). An extensive study by Walther et al. (2013), using about 400 Mucor strains, led to a refinement in the classification of Mucor species. Phylogeny-based on 28S rDNA led to the transfer of some species to different groups and it was shown that some of these groups intermingled with other genera, such as Chaetocladium and Helicostylum, which do not belong to Mucoraceae. The use of five markers (ITS, rpb1, tsr1, mcm7 and cfs) phylogeny by Wagner et al. (2019), combined with phenotypic studies, mating tests and the determination of the maximum growth temperatures revealed 16 phylogenetic species of which 14 showed distinct phenotypical traits and were recognized as discrete species.
Recommended genetic markers (genus level) – LSU and SSU
Recommended genetic markers (species level) – ITS and rpb 1
Accepted number of species – There are 735 species epithets in Index Fungorum (2020), however only 76 species have DNA sequence data (Table 1) (Walther et al. 2019)
References–Larone 1995, Sutton et al. 1998, de Hoog et al. 2000 (morphology); Nguyen et al. 2016, 2017, Walther et al. 2013, 2019, Wagner et al. 2019 (morphology and phylogeny).
Table 1 DNA barcodes available for Mucor. Ex-type/ex-epitype/ex-neotype/ex-lectotype strains are in bold and marked with an asterisk (*). Voucher strains are also in bold. Species confirmed with pathogenicity studies are marked with #.
| Species | Isolate | ITS | LSU |
| Mucor abundans | CBS 388.35 | JN206111 | NG_063979 |
| CBS 521.66 | JN206110 | JN206457 | |
| M. aligarensis | CBS 993.70* | JN206461 | |
| M. ambiguus | CBS 126943 | MH864344 | MH875788 |
| M. amphibiorum | CBS 763.74 | NR_103615 | NG_057877 |
| M. ardhlaengiktus | CBS 210.80* | NR_152960 | JN206504 |
| M. atramentarius | CBS 202.28* | MH854979 | JN206418 |
| M. amethystinus | CBS 846.73* | JN206014 | |
| CBS 526.68 | JN206015 | JN206426 | |
| M. azygosporus | CBS 292.63* | NR_103639 | NG_057928 |
| M. bacilliformis | CBS 251.53* | NR_145285 | NG_057916 |
| M. bainieri | CBS 293.63* | NR_103628 | JN206424 |
| M. brunneogriseus | CBS 129.41* | NR_145283 | JF723735 |
| M. caatinguensis | URM 7322 | NG_060334 | |
| M. circinelloides | B5-2 | KT876701 | |
| CBS 108.16 | JN205954 | ||
| M. ctenidius | CBS 293.66 | MH858796 | JN206417 |
| M. durus | CBS 156.51* | NR_145295 | NG_057918 |
| M. ellipsoideus | ATCC MYA-4767* | NR_111683 | NG_042602 |
| M. endophyticus | CBS 385.95* | NR_111661 | NG_057970 |
| M. exponens | CBS 141.20 | MH854686 | JN206441 |
| M. falcatus | CBS 251.35* | NR_103647 | NG_057931 |
| M. flavus | CBS 230.35* | JN206061 | JN206464 |
| CBS 893.73 | JN206465 | ||
| M. fragilis | EML-PUKI06-1 | KY047147 | |
| M. fuscus | CBS 132.22 | JF723619 | |
| CBS 230.29 | JN206204 | ||
| M. fusiformis | CBS 336.68* | NR_111660 | NG_057915 |
| M. gigasporus | CBS 566.91* | NR_103646 | NG_057926 |
| M. genevensis | CBS 114.08* | HM623318 | |
| CBS 404.71 | JN206042 | ||
| M. guiliermondii | CBS 174.27* | NR_103636 | NG_057923 |
| M. heterogamus | CBS 338.74 | JN206169 | JN206488 |
| CBS 405.58 | JN206167 | ||
| M. hiemalis | CBS 242.35 | JN206134 | |
| CBS 115.18 | JN206127 | ||
| M. inaequisporus | CBS 255.36 | JN206177 | NG_057929 |
| M. indicus | CBS 226.29* | NR_077173 | NG_057878 |
| M. irregularis | CBS 977.68 | JX976259 | |
| EML-PUKI12-1 | KY047151 | ||
| M. japonicus | CBS 154.69 | JN206158 | JN206446 |
| M. koreanus | EML-QT1 | KT936259 | |
| EML-QT2 | KT936260 | ||
| M. lanceolatus | CBS 638.74 | JN206205 | JN206443 |
| M. laxorrhizus | CBS 143.85* | NR_103642 | NG_057914 |
| M. luteus | CBS 243.35 | JX976254 | |
| M. megalocarpus | CBS 215.27* | NR_145286 | NG_057925 |
| M. merdophylus | URM-7908 | MK775467 | MK775466 |
| M. microspores | CBS 204.28 | JN206272 | JN206521 |
| M. minutes | CBS 586.67* | JN206048 | JN206463 |
| M. moelleri | CBS 406.58 | MH858663 | NG_057875 |
| M. mousanensis | CBS 999.70* | NR_103629 | NG_057912 |
| M. mucedo# | CBS 640.67* | NR_103688 | NG_057876 |
| CBS 987.68 | JN206089 | JN206480 | |
| M. multiplex | CBS 110662* | NR_111662 | NG_057924 |
| M. nederlandicus | CBS 735.70 | JN206176 | JN206503 |
| M. nidicola | EML-SBD1 | KY047148 | |
| EML-SBD2 | KY047149 | ||
| M. odoratus | CBS 130.41* | NR_145287 | NG_057927 |
| M. parviseptatus | CBS 417.77 | JN206108 | JN206453 |
| M. piriformis# | CBS 169.25* | NR_103630 | NG_057874 |
| M. plasmaticus | CBS 275.49 | JN206483 | |
| M. plumbeus | CBS 634.74 | HM999955 | HM849677 |
| M. prayagensis | CBS 652.78 | JN206189 | JN206498 |
| M. pseudolusitanicus | CBS 540.78* | MF495059 | |
| CBS 543.80 | MF495060 | ||
| M. pseudocircinelloides | CBS 541.78 | JN206013 | JN206431 |
| M. saturninus | CBS 974.68* | NR_103635 | JN206458 |
| M. stercorarius# | CNUFC-UK2-1* | KX839689 | |
| CNUFC-UK2-2 | KX839680 | ||
| M. strictus# | CBS 576.66* | NR_103631 | |
| M. racemosus | CBS 260.68* | NR_126135 | NG_055727 |
| M. racemosus f. sphaerosporus | CBS 115.08 | JN205919 | JN206433 |
| M. ramosissimus | CBS 135.65* | NR_103627 | NG_056280 |
| M. silvaticus | CBS 249.35 | JN206122 | JN206455 |
| M. ucrainicus | CBS 674.88 | JN206192 | JN206507 |
| M. variisporus | CBS 837.70* | NR_152951 | NG_057972 |
| M. variicolumellatus | CBS 236.35* | JN205979 | JN206422.1 |
| SF012536 | MF495054.1 | ||
| M. velutinosus | UTHSC 04-1961 | JF299208 | |
| M. zonatus | CBS 148.69* | NR_103638 | NG_057917 |
| M. zychae | CBS 416.67* | NR_103641 | NG_057930 |
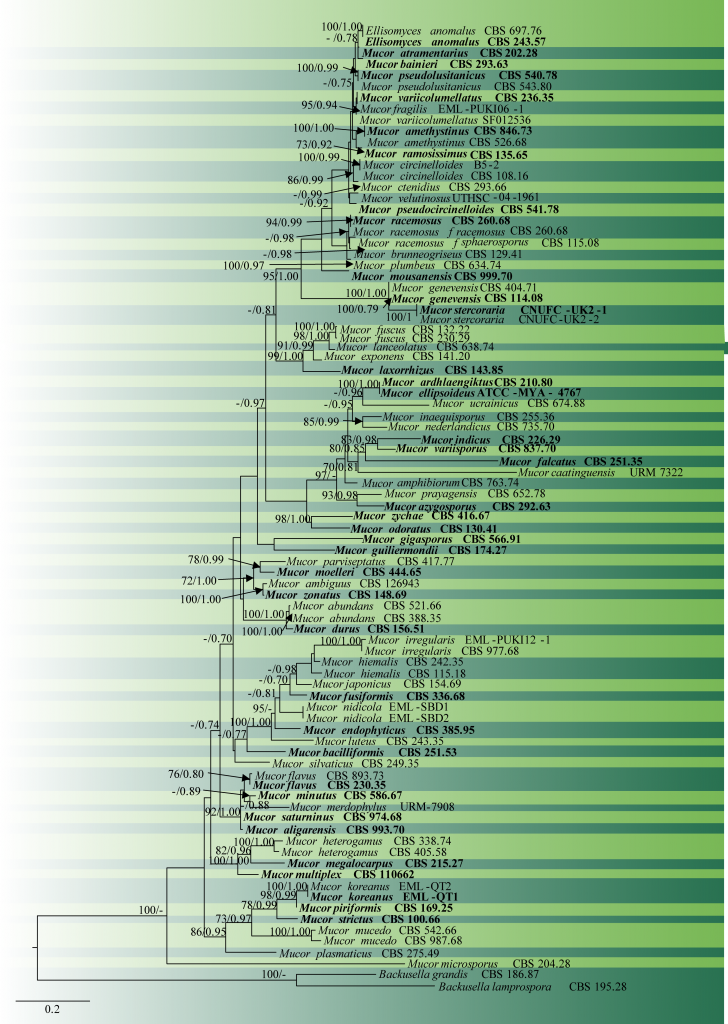
Fig. 1 Phylogram generated from RAxML analysis based on combined sequences of ITS and LSU of Mucor and Backusella species. Eighty-seven taxa were used for the analysis, which consisted of 1264 characters including gaps. The tree is rooted using Backusella lamprospora (CBS 195.28), and B. grandis (CBS 186.87). Likelihood of the best-scoring ML tree was-17553.567209. The concatenated matrix contained 716 distinct alignment patterns with 21.98% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.302082, C = 0.168706, G = 0.219403, T = 0.309809; substitution rates AC = 0.749467, AG = 2.977575, AT = 1.651634, CG = 0.631954, CT = 4.647089, GT = 1.000000; gamma distribution shape parameter α = 0.302610. The type species are in bold. Scale bar indicates the number of substitutions per site. ML bootstrap support values greater than 70% are shown near the nodes.

No Comments