26 Apr Corticiaceae
Corticiaceae Herter, Krypt.-Fl, Brandenburg (Leipzig) 6(1):70 (1910)
Background
Corticiaceae is one of the oldest family names established by Heter (1910) and for a long time was a repository for all basidiomycetes sharing corticioid type of fruiting body, that is basidiomes with resupinate or cortex-like appearance formed over the surface of the substrata. In this traditional sense, Corticiaceae included many distantly related taxa, now shown to be distributed in different orders of basidiomycete phylogeny (Binder et al. 2005). The family name was conserved against Vuilleminiaceae by Pouzar (1985). Corticiaceae taxa are widespread and inhabit a wide range of substrata. Corticiaceae present diverse nutritional habits, with saprotrophic, plant pathogenic, mycoparasitic, lichenized and lichenicolous members. Species of Limonomyces and Waitea are plant pathogens, whereas Erythricium and Laetisaria also include saprotrophic, mycoparasitic, or lichenicolous species. Several species in this family form visible pink fruiting bodies on living or dead plants. The phytopathogenic species are mostly the agent of ‘pink disease’ in turfgrasses or woody perennials. Several taxa in this family are known only as asexual morphs, while plant pathogenic genera are sexual morph-typified and form sexual fruiting bodies, in addition to their asexual state.
Classification – Agaricomycetes, incertae sedis, Corticiales
Type– Corticium Pers., Neues Mag. Bot. 1:110 (1794)
Distribution – Worldwide
Disease Symptoms – Brown ring patch, Pink disease, Pink Patch disease, Red thread Sheath spot.
The symptoms include the production of salmon pink mycelium on branches and stems of trees resulting in twig and branch injuries, stem canker and eventually death of the host (Sebastianes et al. 2007). With sheath spot disease, lesions first appear as water-soaked areas with grey-green to straw coloured centers and a brown margin. Leaves of infected sheaths usually turn yellow and die (Lanoiselet et al. 2007). Circular or irregular small patches of tan to yellow-brown are the initial symptoms of brown ring patch disease. The affected grasses eventually develop brownish rings (Toda et al. 2005). In pink patch and red thread disease, small water-soaked spots covering a larger portion of the grass leaf can be observed. The tissue dries out and fades to a tan colour and is covered with pink mycelium.
Hosts – Citrus sp., Coffea sp., Hevea sp., Poaceae
Morphological based identification and diversity
The 10th edition of Dictionary of Fungi (Kirk et al. 2008), enumerates 29 genera and 136 species associated with Corticiaceae. This figure is, however, outdated as many taxa recorded there do not belong to Corticiaceae, following the most recent phylogenies. The family was delimited in its strict sense by Ghobad-Nejhad et al. (2010). They found that Corticiaceae forms a small, well-supported clade in Corticiales containing several polyphyletic genera in need of revision, and confirmed that it is the most diverse family in Corticiales with regard to its high ecological and nutritional diversity (Lawrey et al. 2008). Corticiaceae currently encompasses about ten genera and about 40 species. A taxonomic and phylogenetic revision of the family is underway (Ghobad-Nejhad et al., unpublished), which is out of the scope of this study. The four genera with plant pathogenic taxa viz., Laetisaria, Limonomyces, Eryhtricium, and Waitea are discussed in the following parts. Tretopileus is briefly noted here.
Tretopileus is a small asexual genus established by Dodge (1946) for a curious fungus found on cactus. The genus is typified by T. opuntiae, and two more species, T. indicus and T. sphaerophorus, were subsequently added to the genus. Okada et al. (1998) showed that T. sphaerophorus was placed in “Aphyllophorales“, But later studies confirmed its position in Corticiales (Rungjindamai et al. 2008), and within Corticiaceae (Ghobad-Nejhad et al. 2010). To date, only T. sphaerophorus has been subject to phylogenetic studies, while the affinities of the generic type as well as T. indicus are yet to be examined. Okada et al. (1998) believe that Tretopileus species may be weakly parasitic on plants.
Most Corticiaceae species form visible pink fruiting bodies on living or dead plants. Basidia are usually large with a swollen base, producing large ellipsoid basidiospores. However, as stated above, the boundaries between genera are perplexing due to overlapping characters.
Molecular based identification and diversity
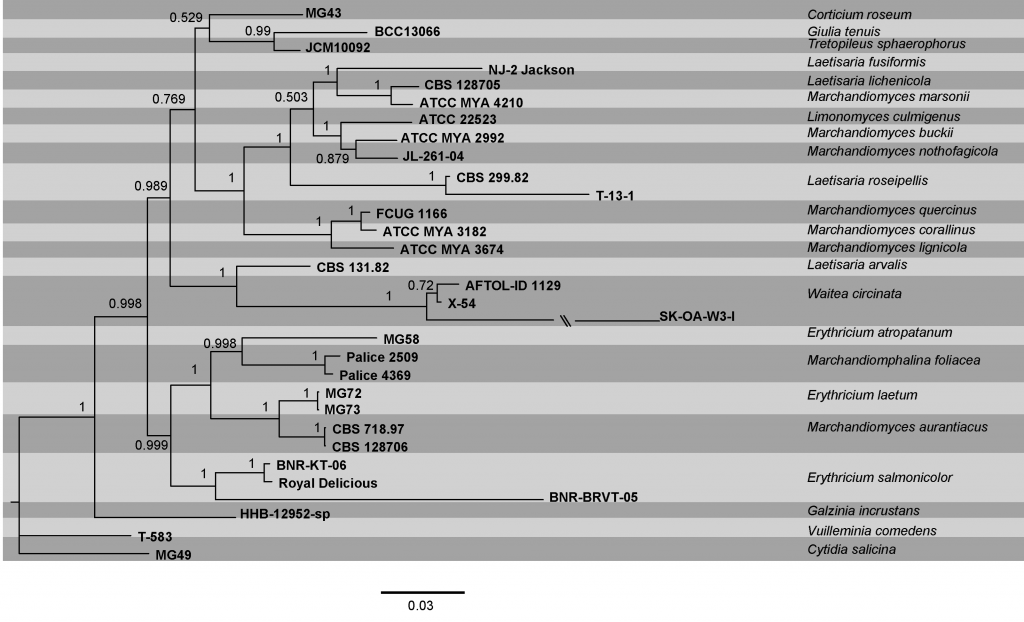
During efforts to unravel Agaricomycetes phylogeny, the lineages belonging to Corticiaceae have been restricted to a small clade more commonly named as ‘corticioid clade’. This clade was formally established as Corticiales by Hibbett et al. (2007). Corticiales was subsequently shown to encompass three well-supported clades recognized by Ghobad-Nejhad et al. (2010) under three family names Corticiaceae, Punctulariaceae and Vuilleminiaceae. Circumscription of most of the genera in Corticiaceae is problematic and synapomorphies for generic and specific delimitations in Corticiaceae are not yet resolved. The phylogenetic tree provided (Fig) is based on a combined dataset of ITS, nSSU, nLSU, and mtSSU sequence data. This phylogenetic tree is largely in accordance with earlier studies and provides the most conclusive phylogeny of the family to date.
Recommended genetic markers (genus level) – LSU, mtSSU
Recommended genetic marker (species level) – ITS
Accepted genera: In this family, ten genera have been accepted, all with DNA molecular data.
References: Binder et al. 2005, Hibbett et al. 2007, Ghobad-Nejhad et al. 2010 (morphology and phylogeny). Toda et al. 2005, Sebastianes et al. 2007 (molecular phylogeny and pathogenicity)
Table Corticiaceae. Details of the isolates used in the phylogenetic analyses. Ex-type (ex-epitype) strains are in bold and marked with an * and voucher strains are in bold.
| Species | Isolate | ITS | nLSU | nSSU | mtSSU |
| Corticium roseum | MG43 | GU590877 | EF537893 a | – | – |
| Cytidia salicina | mg49 | GU590881 | HM046921 | – | AF214458a |
| Erythricium atropatanum | MG58* | GU590876 | GU590880 | – | – |
| E. laetum | MG72 | GU590875 | GU590878 | – | – |
| E. laetum | MG73 | GU590874 | GU590879 | – | – |
| E. salmonicolor | BNR-KT-06 | EU435008 | AY672680a | – | – |
| E. salmonicolor | BNR-BRVT-05 | EU435009 | AY672678a | – | – |
| E. salmonicolor | Royal Delicious | KF029722 | KF029722 | – | – |
| Galzinia incrustans | HHB-12952-sp | – | AF518617 | AF518578 | AF518679 |
| Giulia tenuis | BCC13066 | – | EF589739 | EF589732 | – |
| Laetisaria arvalis | CBS 131.82* | EU622841 a | EU622842 | EU622843 | HQ168390 |
| L. fuciformis | NJ-2 Jackson | EU118639 | AY293192 | AY293139 | AY293232 |
| L. lichenicola | CBS 128705* | NR_121484 | HQ168400 | HQ168399 | HQ168389 |
| Limonomyces culmigenus | ATCC 22523 | EU622849 | EU622848 | EU622847 | – |
| L. roseipellis | CBS 299.82 | EU622846 | EU622844 | EU622845 | HQ168396 |
| L. roseipellis | T-13-1 | KC193592 | KF824726a | AY613915a | KF824721a |
| Marchandiomyces aurantiacus | CBS 718.97 | AY583324 | AY583330 | DQ915460a | – |
| M. aurantiacus | CBS 128706 | HQ168397 | HQ168397 | HQ168398 | HQ168388 |
| Marchandiomyces buckii | ATCC MYA 2992 (JL244-03)* | – | DQ915472 | DQ915462 | HQ168392 |
| M. corallinus | ATCC MYA 3182 | AY583327a | AY583331 a | DQ915464 | HQ168393 |
| M. lignicola | ATCC MYA 3674 | FJ172272 a | AY583332 a | DQ915465 | HQ168391 |
| M. marsonii | ATCC MYA 4210* | EU622840 | EU622839 | EU622838 | HQ168395 |
| M. nothofagicola | JL-261-04 | DQ915474 | DQ915474 | DQ915466 | HQ168394 |
| M. quercinus | FCUG1166 | KP864659b | HM046929a | – | – |
| Marchandiomphalina foliacea | Palice 4369 | AY542865 | AY542865 | AY542865 | – |
| M. foliacea | Palice 2509 | AY542864 | AY542864 | AY542864 | – |
| Tretopileus sphaerophorus | JCM10092 | – | – | AB006005 | – |
| Vuilleminia comedens | T-583 | HM046880a | AF518666 | AF518594 | AF518699 |
| Waitea circinata | AFTOL-ID 1129 | DQ356414a | AY885164 | – | FJ440234 a |
| W. circinata | SK-OA-W3-I | HM597147 | AD001658 a | – | FJ440232 a |
| W. circinata | X-54 | KC176341 | KC176341 | – | FJ440221a |
a Sequences obtained from a different isolate
b Newly generated sequence
Fig. Phylogram generated from bayesian analysis based on combined ITS, nSSU, nLSU, and mtSSU sequence data of Corticiaceae. Bayesian posterior probabilities are indicated above the nodes. The sequences were obtained from GenBank. Thirty one isolates were included in the analyses, comprising 3797 characters including gaps. The tree obtained from Bayesian analyses with the average standard deviation of split frequency equal to 0.005882 is presented. Among these, 2585 characters were constant, and 550 characters were variable but parsimony uninformative. The analyses run for 30 million generations and 8 MCMCMC chains, with 5000 sample frequency. The ITS partition was analysed with GTR+G model of nucleotide evolution, while the nSSU, nLSU, and mtSSU datasets were analysed using GTR+I+G model, as suggested by MrModeltest. The ex-type (ex-epitype) and voucher strains are in bold. The scale bar indicates 0.03 changes. The tree is rooted with Cytidia salicina.

No Comments