20 Sep Bipolaris
Bipolaris Shoemaker, Can. J. Bot. 33:882(1959)
For synonyms see Index Fungorum (2018)
Background
Species of Bipolaris are cosmopolitan and distributed throughout a broad range of environments. Bipolaris species are pathogens, saprobes or endophytes of a wide range of hosts (Hyde et al. 2014). Bipolaris oryzae critically damaged the rice cultivation in Bengal province in India and caused a devastating famine during 1943–1944 (Scheffer 1997; Hyde et al. 2014). Although not resulting in human starvation, Southern corn leaf blight caused by Bipolaris maydis in the 1970s resulted in catastrophic losses in maize crops in the USA and UK (Manamgoda et al. 2014). Bipolaris sorokiniana was confirmed as the most economically important foliar pathogen in warm areas in the conference “Wheat for the national warm areas” held in Brazil in 1990 (Hyde et al. 2014). Some Bipolaris species are pathogenic to humans (El-Khizzi et al. 2010). Transferring agricultural commodities including plants and seeds across geographical borders without proper quarantine implementation may have resulted in the worldwide distribution of common phytopathogenic species of Bipolaris (Farr and Rossman 2018; Manamgoda et al. 2014).
Classification – Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae
Type species – Bipolaris maydis (Y.Nisik. & C. Miyake) Shoemaker, Can. J. Bot. 33:882(1959)
Distribution – Worldwide
Disease Symptoms –Leaf spots, leaf blights, melting outs, common root rot, foot rot
Small brown-red water-soaked spots on leaves can be observed. Subsequently, the disease area may turn into black/brown elliptical or fusiform lesions with grey to brown centers. On a fully developed lesion concentric rings can be observed (Lin et al. 2012; Ahmadpour et al. 2012). Decaying leaves with purple/brown lesions are observed in melting out disease (Watkins 1989). Brown to black lesions on primary and secondary roots, brown discolouration of crowns, yellowing of plants and browning of sub-crown internode can be observed in common root rot disease (Arabi et al. 2013). In foot rot disease, dark brown lesions on the sub-crown are caused. These lesions will eventually spread to encompass the entire sub-crown internode (Smiley and Patterson 1996).
Hosts – Poaceae, including rice, maize, wheat and sorghum (Manamgoda et al. 2014). Species of Bipolaris are also recorded from at least 60 other plant genera in Anacardiaceae, Araceae, Euphorbiaceae, Fabaceae, Malvaceae, Rutaceae and Zingiberaceae as either saprobes or pathogens (Manamgoda et al. 2011; Ariyawansa et al. 2015a).
Morphological based identification and diversity
Correct species identification in this genus has always proven difficult, mostly relying on morphology and plant host association. Studies on the morphology of the sexual morph of most Bipolaris are lacking due to difficulties to induce this morph in culture or to find it in nature (Manamgoda et al. 2014). Manamgoda et al. (2014) revised the genus based on DNA sequence data derived from living cultures of fresh isolates, available ex-type cultures from worldwide collections and observation of type and additional specimens. They accepted 47 species in Bipolaris and clarified the taxonomy, host associations, geographic distributions and species synonymy while epi- or neotypes were designated (Ariyawansa et al. 2015a). Currently, there are 131 species epithets in Index Fungorum (www.indexfungorum.org; retrieved 24 March 2018). Wijayawardene et al. (2017b) estimated there were 121 species in this genus. In a recent study Marin-Felix et al. (2017) has included 40 accepted Bipolaris species to their phylogenetic analyses. To properly delineate these species, phylogenetic studies using ITS, GAPDH and TEF1- α sequences were recently performed (Manamgoda et al. 2014, 2015; Marin-Felix et al. 2017).
Identification based on morphology alone is imperfect since many species have overlapping characters. The genus is morphologically similar to Curvularia and distinguishing these two genera can be problematic (Manamgoda et al. 2011, 2014). Both genera contain species with straight or curved conidia, but in Bipolaris the curvature is continuous throughout the length of the conidium, while the conidia of Curvularia have intermediate cells inordinately enlarged which contributes to their curvature (Manamgoda et al. 2011, 2014; Marin-Felix et al. 2017). Conidia in Bipolaris are usually longer than in Curvularia. Also, the presence of stromata in some species of Curvularia is significant whereas this feature is not observed in Bipolaris (Manamgoda et al. 2014; Marin-Felix et al. 2017).
Molecular based identification and diversity
To achieve better generic and species delimitation, phylogenetic studies using ITS, GAPDH and TEF1-α were recently performed (Manamgoda et al. 2014; Hyde et al. 2014; Marin-Felix et al. 2017). Phylogenetic studies based on these loci made it possible to reallocate species of Cochliobolus (sexual morph) to either Bipolaris or Curvularia (Marin-Felix et al. 2017). We update the phylogeny of Bipolaris based on analyses of a combined ITS, GAPDH and TEF1-α sequence data and it is in accordance with previous studies. Sequences obtained were from available ex-epitype, ex-isotype, ex-isolectotype, ex-paratype, ex-syntype and ex-type strains cultures.
Recommended genetic marker (genus level) – LSU
Recommended genetic markers (species level) – ITS, GAPDH and TEF
Accepted number of species: There are 130 species epithets in Index Fungorum (2018) under this genus. However, only 40 are accepted.
References: Manamgoda et al. 2011, 2014, Ariyawansa et al. 2015a, Marin-Felix et al. 2017 (morphology, phylogeny).
Table Bipolaris. Details of the isolates used in the phylogenetic analyses. Ex-type (ex-epitype) strains are in bold and marked with an * and voucher strains are in bold.
| Species | Isolate | ITS | GAPDH | TEF1- α |
| Bipolaris austrostipae | BRIP 12490* | KX452442 | KX452408 | KX452459 |
| B. axonopicola | BRIP 11740* | KX452443 | KX452409 | KX452460 |
| B. bamagaensis | BRIP 13577* | KX452445 | KX452411 | KX452462 |
| B. bicolour | CPC 28811 | MF490804 | MF490826 | MF490848 |
| B. bicolour | CPC 28825 | MF490805 | MF490827 | MF490849 |
| B. bicolour | CBS 690.96 | KJ909762 | KM042893 | KM093776 |
| B. brachiariae | CPC 28819* | MF490806 | MF490828 | MF490850 |
| B. brachiariae | CPC 28820 | MF490807 | MF490829 | MF490851 |
| B. chloridis | BRIP 10965* | KJ415523 | KJ415423 | KJ415472 |
| B. clavata | BRIP 12530* | KJ415524 | KJ415422 | KJ415471 |
| B. coffeana | BRIP 14845* | KJ415525 | KJ415421 | KJ415470 |
| B. cookie | AR 5185 | KJ922391 | KM034833 | KM093777 |
| B. crotonis | BRIP 14838 | KJ415526 | KJ415420 | KJ415479 |
| B. cynodontis | CBS 109894 | KJ909767 | KM034838 | KM093782 |
| B. drechsleri | CBS 136207* | KF500530 | KF500533 | KM093760 |
| B. gossypina | BRIP 14840* | KJ415528 | KJ415418 | KJ415467 |
| B. heliconiae | BRIP 17186* | KJ415530 | KJ415417 | KJ415465 |
| B. heveae | CBS 241.92 | KJ909763 | KM034843 | KM093791 |
| B. luttrellii | BRIP 14643* | AF071350 | AF081402 | AF071350 |
| B. maydis | CPC 28823 | MF490808 | MF490830 | MF490852 |
| B. maydis | CBS 137271* | AF071325 | KM034846 | KM093794 |
| B. microlaenae | CBS 280.91* | JN601032 | JN600974 | JN601017 |
| B. microstegii | CBS 132550* | JX089579 | JX089575 | KM093756 |
| B. oryzae | CPC 28826 | MF490809 | MF490831 | MF490853 |
| B. oryzae | CPC 28828 | MF490810 | MF490832 | MF490854 |
| B. oryzae | MFLUCC 10-0715* | JX256416 | JX276430 | JX266585 |
| B. panici-miliacei | CBS 199.29* | KJ909773 | KM042896 | KM093788 |
| B. peregianensis | BRIP 12790* | JN601034 | JN600977 | JN601022 |
| B. pluriseptata | BRIP 14839* | KJ415532 | KJ415414 | KJ415461 |
| B. sacchari | ICMP 6227 | KJ922386 | KM034842 | KM093785 |
| B. saccharicola | CBS 155.26* | KY905674 | KY905686 | KY905694 |
| B. saccharicola | CBS 324.64 | HE792932 | KY905692 | KY905699 |
| B. saccharicola | CBS 325.64 | KY905675 | KY905687 | KY905695 |
| B. salkadehensis | Bi 1* | AB675490 | AB675490 | AB675490 |
| B. salviniae | BRIP 16571* | KJ415535 | KJ415411 | KJ415457 |
| B. secalis | BRIP 14453* | KJ415537 | KJ415409 | KJ415455 |
| B. setariae | CPC 28802 | MF490811 | MF490833 | MF490811 |
| B. setariae | CBS 141.31 | EF452444 | EF513206 | EF452444 |
| B. shoemaker | BRIP 15929* | KX452453 | KX452419 | KX452470 |
| B. simmondsii | BRIP 12030* | KX452454 | KX452420 | KX452471 |
| B. sivanesaniana | BRIP 15847* | KX452455 | KX452421 | KX452472 |
| B. sorokiniana | CPC 28832 | MF490812 | MF490834 | MF490855 |
| B. sorokiniana | CBS 110.14 | KJ922381 | KM034822 | KM093763 |
| B. subramanianii | BRIP 16226* | KX452457 | KX452423 | KX452474 |
| B. urochloae | ATCC 58317 | KJ922389 | KM230396 | KM093770 |
| B. variabilis | CBS 127716* | KY905676 | KY905688 | KY905696 |
| B. variabilis | CBS 127736 | KY905677 | KY905689 | KY905677 |
| B. victoriae | CBS 327.64* | KJ909778 | KM034811 | KM093748 |
| B. woodii | BRIP 12239* | KX452458 | KX452424 | KX452475 |
| B. yamadae | CPC 28807 | MF490813 | MF490835 | MF490856 |
| B. yamadae | CBS 202.29* | KJ909779 | KM034830 | KM093773 |
| B. yamadae | CBS 127087 (neotype of B. euphorbiae) | KY905673 | KY905685 | KY905693 |
| B. zeae | BRIP 11512IsoP* | KJ415538 | KJ415408 | KJ415454 |
| B. zeicola | FIP 532* | KM230398 | KM034815 | KM093752 |
| Curvularia lunata | CBS 730.96* | JX256429 | JX276441 | JX266596 |
| C. sorghina | BRIP 15900* | KJ415558 | KJ415388 | KJ415435 |
| C. subpapendorfii | CBS 656.74* | KJ909777 | KM061791 | KM196585 |
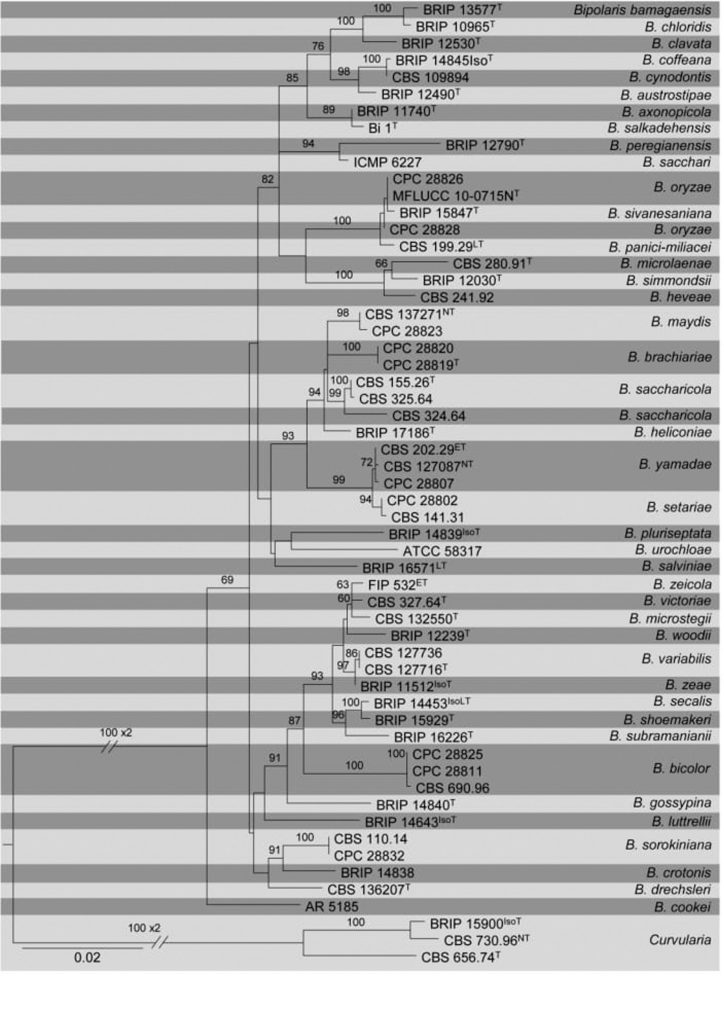
Fig Phylogenetic tree generated by maximum likelihood analysis of combined ITS, GAPDH and TEF1- α sequence data of Bipolaris species. Related sequences were obtained from GenBank. Fifty-seven strains are included in the analyses, which comprises 1998 characters including gaps. Tree was rooted with Curvularia subpapendorfii (CBS 656.74), C. lunata (CBS 730.96) and C. sorghina (BRIP 15900). The best scoring RAxML tree with a final likelihood value of -7757.628604 is presented. The matrix had 492 distinct alignment patterns, with 10.59% of undetermined characters or gaps. Estimated base frequencies were as follows; A = 0.232326, C = 0.300959, G = 0.235960, T = 0.230755; substitution rates AC = 0.597533, AG = 2.227202, AT = 0.791120, CG = 0.627286, CT = 4.571166, GT = 1.000000; gamma distribution shape parameter α = 0.779269. RAxML bootstrap support values ≥60% (BT) are shown respectively near the nodes. The scale bar indicates 0.02 changes. T, ET, IsoT, IsoLT, IsoPT, LT and NT indicate ex-type, ex-epitype, ex-isotype, ex-isolectotype, ex-isoparatype, ex-lectotype and ex-neotype strains, respectively.

No Comments