27 Oct Cladosporium
Cladosporium Link, Mag. Gesell. naturf. Freunde, Berlin 7: 37 (1816) [1815]
Background
Cladosporium belongs to Cladosporiaceae in the order Capnodiales (Hyde et al. 2013; Liu et al. 2017). Established in 1816 with C. herbarum as type species, Cladosporium is one of the largest genera of dematiaceous hyphomycetes. Davidiella was erected by Braun et al. (2003) to accommodate the sexual morph of Cladosporium sensu stricto. Davidiella was therefore recognized as a synonym of Cladosporium as Cladosporium has priority over Davidiella at generic rank, and is also the more commonly used name in literature (Bensch et al. 2012). Therefore, Cladosporiaceae took preference over Davidiellaceae (Bensch et al. 2012). Cladosporium species have a worldwide distribution and can be easily spread in the environment, because of their small conidia. Cladosporium includes many important pathogens causing leaf spots and stem rots of many plant hosts. For example, Cladosporium fulvum is the causal agent of tomato leaf mold (van Kan et al. 1991). Cladosporium species have been recorded as endophytes and may have a positive effect, for example, C. sphaerospermum was isolated from the roots of Glycine max which can promote its growth (Hamayun et al. 2009). Some species, such as C. herbarum, are also known as common contaminants in clinical laboratories and cause allergic lung disease (de Hoog et al. 2000). Several species were also isolated from human respiratory samples (Sandoval-Denis et al. 2016). Thirteen species are fungicolous (Heuchert et al. 2005; Sun et al. 2019) and have the potential for biological control in agriculture and forestry (Torres et al. 2017).
There have been studies towards understanding the genetic components of Cladosporium. Cladosporium fulvum is an important model species in the plant pathology study. Iakovidis et al. (2020) reported classical mapping strategies for loci of tomato that response to sequence-monomorphic effector Ecp5. Convergent evolution could be used for choosing different functional genes according to individual plant breeding needs. Ge et al. (2019) showed that Cladosporium species have the potential to be used in industrial processes. They identified a new glucose oxidase gene CtgoxB from C. tianshanense and suggested this could be a candidate for the aquatic feed and detergent industries. Transcriptome and proteome analyses of C. fulvuim showed that 14 out of 59 predicted proteases are expressed during in vitro and in planta, of which nine belong to serine proteases and the rest belong to metallo and aspartic proteases (Jashni et al. 2019). This study also confirmed the presence of six proteases at proteome level during the infection.
Grinn-Gofroń et al. (2019) developed and evaluated the models of forecasting possibilities of airborne spore concentrations in 18 sites in six countries across Europe. The study revealed the possibility of reliable prediction of fungal spore levels using gridded meteorological data. They concluded that these forecasting models can be used in the more timely and efficient management of phytopathogenic and of human allergic diseases. An environmentally isolated strain of C. sphaerospoermum substantially enhanced plant growth, early flowering and increase in crop yield after exposure in vitro (Li et al. 2019). Pan et al. (2020) identified four new hybrid polyketides (Cladosin L-O) from C. shaerospermum which showed strong cytotoxicity, antifungal activity and moderate antibacterial activity.
Classification: Ascomycota, Pezizomycotina, Dothideomycetes, Pleosporomycetidae, Capnodiales, Cladosporiaceae
Type species–Cladosporium herbarum (Pers.) Link
Distribution– Worldwide
Disease symptoms–Leaf spots, leaf blight, discolourations, necrosis, or shot-hole symptoms, on stems and fruits, rots
Hosts– Cladosporium species occur on a wide range of host plants including Asparagaceae, Asteraceae, Fabaceae, Myrtaceae, Orchidaceae, Poaceae, Solanaceae and Vitaceae (Farr and Rossman 2020). Some species can be hyperparasites of insects and fungi (Heuchert et al. 2005; Islam et al. 2019; Sun et al. 2019; Abdel-Baky 2000). These species can cause allergies in humans such as sneezing, hives and also can cause eye, ear and sinus infections (de Hoog et al. 2000).
Pathogen biology, disease cycle and epidemiology
Cladosporium survives in the soil or on plant debris and produce spores during humid weather. Fungal spores germinate under high humidity and cool to warm temperatures. Wind, rain and irrigation splash, workers, tools, and insects readily disseminate spores (Jordan et al. 1990; Lan and Scherm 2003; Liu et al. 2019).
Morphological based identification and diversity
The asexual morph of Cladosporium species is characterized by a unique coronate structure of the conidiogenous loci and conidia, consisting of the central convex dome surrounded by a raised periclinal rim (Bensch et al. 2012; Fig 1), while ascomata of sexual morphs are identical to those of Mycosphaerella (sect. Tassiana) (Braun et al. 2003). Historically, all types of dematiaceous hyphomycetes with amero- to phragmosporous conidia formed in acropetal chains had been assigned to Cladosporium sensu lato, resulting in the complication to resolve a natural classification of Cladosporium. Various mycologists proposed natural genetic circumscriptions of Cladosporium (David 1997; Braun et al. 2003; Aptroot 2006). David (1997) found the unique structure of conidiogenous loci and conidial hila using scanning electron microscopy. Based on the genetic circumscriptions, some cladosporioid groups, such as Fusicladium being non-coronate (Schubert et al. 2003), have been excluded from Cladosporium s. str. Various Cladosporium species have been re-examined based on the new generic concepts (Schubert and Braun 2004, 2005a, b, 2007; Schubert 2005; Schubert et al. 2006; Braun and Schubert 2007; Braun et al. 2008). A polyphasic approach revealed three major species complexes within Cladosporium, viz. C. cladosporioides, C. herbarum and C. sphaerospermum (Schubert et al. 2007; Dugan et al. 2008; Bensch et al. 2010; Bensch et al. 2015). A modern monograph of the genus treated 993 names of Cladosporium sensu lato, of which 169 were recognized in Cladosporium sensu stricto and others remain doubtful (Bensch et al. (2012).
Fig. 1 Cladosporium cladosporioides.a. Conidiomata. b-c,e. Macro- and micronematous conidiophores and conidia chains. d. Secondary ramoconidia. f. Conidia. Scale bars: b–c, e–f=50 µm, d–g=10 µm.
Molecular based identification and diversity
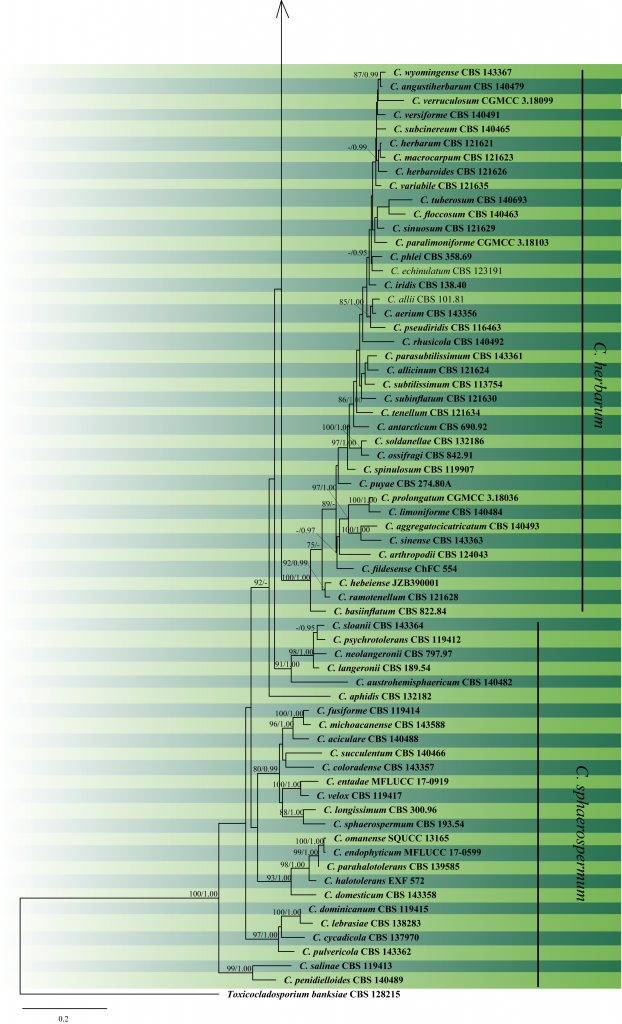
The first molecular examination of Cladosporium-like hyphomycetes based on ITS and SSU was carried out by Braun et al. (2003), who confirmed the strong heterogeneity. A new genus Davidiella was established to accommodate the sexual morphs of Cladosporium sensu stricto species which were previously assigned in Mycosphaerella. Aptroot (2006) made a better circumscription of Davidiella after he found species of Davidiella have ascospores with irregular cellular inclusions, which are absent in Mycosphaerella. Schoch et al. (2006) studied the phylogenetic relationships of 96 taxa of the Dothideomycetes using LSU, SSU, tef1 and rpb2 gene data. Davidiella and its Cladosporium asexual morphs were assigned to the family Cladosporiaceae in the order Capnodiales, together with Mycosphaerellaceae. Crous et al. (2007) delimited Cladosporium from morphologically similar genera using their morphology and DNA phylogeny based on LSU. Several species were transferred to new genera such as Hyalodendriella, Ochrocladosporium, Rachicladosporium, Rhizocladosporium, Toxicocladosporium and Verrucocladosporium. Furthermore, C. castellanii was confirmed as a synonym of Stenella araguata, while the type species of Stenella resided in Teratosphaeriaceae instead of Mycosphaerellaceae. Schubert et al. (2007) performed a comprehensive study of the C. herbarum species complex based on both morphology and phylogenetic analysis with five combined genes. Bensch et al. (2010) carried out species and ecological diversity within the C. cladosporioides species complex. More than 200 isolates belonging to the C. cladosporioides species complex were examined and analyzed on the basis of ITS, actand tef1 gene regions. A comprehensive monograph of Cladosporium sensu lato was provided by Bensch et al. (2012) based on morphology and combined ITS, act and tef1 sequence data. In their study, 993 names assigned to Cladosporium sensu lato are treated and 169 names were recognized in Cladosporium sensu stricto. Bensch et al. (2015) introduced the three major species complexes in Cladosporium, i.e. C. cladosporioides, C. herbarum and C. sphaerospermum, and 19 new species were described. Razafinarivo et al. (2016) introduced a new species C. lebrasiae from milk bread rolls in France, Ma et al. (2017) introduced six new soil-inhabiting Cladosporium species from plateaus in China. Bensch et al. (2018) studied Cladosporium species from indoor environments and introduced 16 new species. Several new Cladosporium species including Cladosporium omanense (Halo et al. 2019), C. passiflorae and C. passifloricola (Rosado et al. 2019) have been introduced more recently. In this study, we reconstruct the phylogeny of Cladosporium based on ITS, tef1 and act sequenced data (Table 1; Fig 2).
Recommended genetic marker (genus level) – ITS and LSU
Recommended genetic markers (species level) – act and tef1 (in a few cases tub2)
Accepted number of species–There are 844 epithets listed in Index Fungorum (2020), however, 138 species have DNA sequence data.
References–David 1997, Aptroot 2006, Schubert and Braun 2004, 2005a, b, 2007; Schubert 2005; Schubert et al. 2006; Braun and Schubert 2007; Braun et al. 2008 (morphology); Braun et al. 2003,Schoch et al. 2006, Bensch et al. 2010, 2012, 2015, Ma et al. 2017 (morphology and phylogeny).
Table 1 DNA barcodes available for Cladosporium. Ex-type/ex-epitype/ex-neotype/ex-lectotype strains are in bold and marked with an asterisk (*). Voucher strains are also in bold. Species confirmed with pathogenicity studies are marked with #.
| Species | Isolate | ITS | tef1 | act |
| Cladosporium acalyphae | CBS 125982* | HM147994 | HM148235 | HM148481 |
| C. aciculare | CBS 140488* | KT600411 | KT600509 | KT600607 |
| C. aerium | CBS 143356* | MF472897 | MF473324 | MF473747 |
| C. aggregatocicatricatum# | CBS 140493* | KT600448 | KT600547 | KT600645 |
| C. alboflavescens | CBS 140690* | LN834420 | LN834516 | LN834604 |
| C. allicinum | CBS 121624* | EF679350 | EF679425 | EF679502 |
| C. allii | CBS 101.81 | JN906977 | JN906983 | JN906996 |
| C. angulosum | CBS 140692* | LN834425 | LN834521 | LN834609 |
| C. angustiherbarum | CBS 140479* | KT600378 | KT600475 | KT600574 |
| C. angustisporum | CBS 125983* | HM147995 | HM148236 | HM148482 |
| C. angustiterminale | CBS 140480* | KT600379 | KT600476 | KT600575 |
| C. antarcticum | CBS 690.92* | EF679334 | EF679405 | EF679484 |
| C. anthropophilum | CBS 140685* | LN834437 | LN834533 | LN834621 |
| C. aphidis | CBS 132182* | JN906978 | JN906984 | JN906997 |
| C. arthropodii | CBS 124043* | JN906979 | JN906985 | JN906998 |
| C. asperulatum# | CBS 126340* | HM147998 | HM148239 | HM148485 |
| C. australiense | CBS 125984* | HM147999 | HM148240 | HM148486 |
| C. austroafricanum | CBS 140481* | KT600381 | KT600478 | KT600577 |
| C. austrohemisphaericum | CBS 140482* | KT600382 | KT600479 | KT600578 |
| C. basiinflatum# | CBS 822.84* | HM148000 | HM148241 | HM148487 |
| C. chalastosporoides | CBS 125985* | HM148001 | HM148242 | HM148488 |
| C. chasmanthicola | CBS 142612* | KY646221 | KY646227 | KY646224 |
| C. chubutense | CBS 124457* | FJ936158 | FJ936161 | FJ936165 |
| C. cladosporioides# | CBS 112388* | HM148003 | HM148244 | HM148490 |
| C. colocasiae# | CBS 386.64* | HM148067 | HM148310 | HM148555 |
| C. colombiae | CBS 274.80B* | FJ936159 | FJ936163 | FJ936166 |
| C. coloradense | CBS 143357* | MF472945 | MF473372 | MF473795 |
| C. crousii | CBS 140686* | LN834431 | LN834527 | LN834615 |
| C. cucumerinum# | CBS 171.52* | HM148072 | HM148316 | HM148561 |
| C. cycadicola | CBS 137970* | KJ869122 | KJ869236 | KJ869227 |
| C. delicatulum | CBS 126344* | HM148081 | HM148325 | HM148570 |
| C. domesticum | CBS 143358* | MF472955 | MF473382 | MF473805 |
| C. dominicanum | CBS 119415* | DQ780353 | JN906986 | EF101368 |
| C. echinulatum# | CBS 123191 | JN906980 | JN906987 | JN906999 |
| C. endophyticum | MFLUCC 17-0599* | MG646956 | MG646988 | |
| C. entadae | MFLUCC 17-0919* | MK347728 | ||
| C. europaeum | CBS 134914* | HM148056 | HM148298 | HM148543 |
| C. exasperatum | CBS 125986* | HM148090 | HM148334 | HM148579 |
| C. exile | CBS 125987* | HM148091 | HM148335 | HM148580 |
| C. fildesense | ChFC-554* | JX845290 | MN233633 | MN233632 |
| C. flabelliforme | CBS 126345* | HM148092 | HM148336 | HM148581 |
| C. flavovirens | CBS 140462* | LN834440 | LN834536 | LN834624 |
| C. floccosum# | CBS 140463* | LN834416 | LN834512 | LN834600 |
| C. funiculosum | CBS 122129* | HM148094 | HM148338 | HM148583 |
| C. fusiforme | CBS 119414* | DQ780388 | JN906988 | EF101372 |
| C. gamsianum | CBS 125989* | HM148095 | HM148339 | HM148584 |
| C. globisporum# | CBS 812.96* | HM148096 | HM148340 | HM148585 |
| C. grevilleae | CBS 114271* | JF770450 | JF770472 | JF770473 |
| C. halotolerans | CBS 119416* | DQ780364 | JN906989 | EF101397 |
| C. hebeiense# | JZB390001* | MG516597 | MG516595 | MG516593 |
| C. herbaroides# | CBS 121626* | EF679357 | EF679432 | EF679509 |
| C. herbarum# | CBS 121621* | EF679363 | EF679440 | EF679516 |
| C. hillianum | CBS 125988* | HM148097 | HM148341 | HM148586 |
| C. inversicolor | CBS 401.80* | HM148101 | HM148345 | HM148590 |
| C. ipereniae | CBS 140483* | KT600394 | KT600491 | KT600589 |
| C. iranicum# | CBS 126346* | HM148110 | HM148354 | HM148599 |
| C. iridis# | CBS 138.40* | EF679370 | EF679447 | EF679523 |
| C. kenpeggii# | CBS 142613* | KY646222 | KY646228 | KY646225 |
| C. langeronii | CBS 189.54* | DQ780379 | JN906990 | EF101357 |
| C. lebrasiae | CBS 138283* | KJ596568 | KJ596583 | KJ596631 |
| C. licheniphilum | CBS 125990* | HM148111 | HM148355 | HM148600 |
| C. limoniforme# | CBS 140484* | KT600397 | KT600494 | KT600592 |
| C. longicatenatum | CBS 140485* | KT600403 | KT600500 | KT600598 |
| C. longissimum | CBS 300.96* | DQ780352 | EU570259 | EF101385 |
| C. lycoperdinum | CBS 126347 | HM148112 | HM148356 | HM148601 |
| C. macrocarpum# | CBS 121623* | EF679375 | EF679453 | EF679529 |
| C. magnoliigena | MFLUCC 18-1559* | MK347813 | MK340864 | |
| C. michoacanense | CBS 143588* | LT907958 | LT907945 | LT907961 |
| C. montecillanum | CBS 140486* | KT600406 | KT600504 | KT600602 |
| C. myrtacearum | CBS 126350* | HM148117 | HM148361 | HM148606 |
| C. needhamense | CBS 143359* | MF473142 | MF473570 | MF473991 |
| C. neerlandicum | CBS 143360* | KP701887 | KP701764 | KP702010 |
| C. neolangeronii | CBS 797.97* | MF473143 | MF473992 | |
| C. neopsychrotolerans | CGMCC 3.18031* | KX938383 | KX938400 | KX938366 |
| C. omanense | SQUCC 13165* | MH725789 | MH716047 | MH716046 |
| C. ossifragi | CBS 842.91* | EF679381 | EF679459 | EF679535 |
| C. oxysporum# | CBS 125991 | HM148118 | HM148362 | HM148607 |
| C. paracladosporioides | CBS 171.54* | HM148120 | HM148364 | HM148609 |
| C. parahalotolerans | CBS 139585* | KP701955 | KP701832 | KP702077 |
| C. paralimoniforme | CGMCC 3.18103* | KX938392 | KX938409 | KX938375 |
| C. parapenidielloides | CBS 140487* | KT600410 | KT600508 | KT600606 |
| C. parasubtilissimum | CBS 143361* | MF473170 | MF473593 | MF474018 |
| C. passiflorae# | COAD 2135* | MH682175 | MH724943 | MH729795 |
| C. passifloricola | COAD 2140* | MH724948 | MH729800 | |
| C. penidielloides | CBS 140489* | KT600412 | KT600510 | KT600608 |
| C. perangustum# | CBS 125996* | HM148121 | HM148365 | HM148610 |
| C. phaenocomae | CBS 128769* | JF499837 | JF499875 | JF499881 |
| C. phlei# | CBS 358.69* | JN906981 | JN906991 | JN907000 |
| C. phyllactiniicola | CBS 126352* | HM148150 | HM148394 | HM148639 |
| C. phyllophilum | CBS 125992* | HM148154 | HM148398 | HM148643 |
| C. pini-ponderosae | CBS 124456* | FJ936160 | FJ936164 | FJ936167 |
| C. prolongatum | CGMCC 3.18036* | KX938394 | KX938411 | KX938377 |
| C. pseudiridis | CBS 116463* | EF679383 | EF679461 | EF679537 |
| C. pseudochalastosporoides | CBS 140490* | KT600415 | KT600513 | KT600611 |
| C. pseudocladosporioides# | CBS 125993* | HM148158 | HM148402 | HM148647 |
| C. psychrotolerans | CBS 119412* | DQ780386 | JN906992 | EF101365 |
| C. pulvericola | CBS 143362* | MF473226 | MF473648 | MF474075 |
| C. puyae | CBS 274.80A* | KT600418 | KT600516 | KT600614 |
| C. ramotenellum# | CBS 121628* | EF679384 | EF679462 | EF679538 |
| C. rectoides | CBS 125994* | HM148193 | HM148438 | HM148683 |
| C. rhusicola | CBS 140492* | KT600440 | KT600539 | KT600637 |
| C. ruguloflabelliforme | CBS 140494* | KT600458 | KT600557 | KT600655 |
| C. rugulovarians | CBS 140495* | KT600459 | KT600558 | KT600656 |
| C. salinae | CBS 119413* | DQ780374 | JN906993 | EF101390 |
| C. scabrellum | CBS 126358* | HM148195 | HM148440 | HM148685 |
| C. silenes | CBS 109082* | EF679354 | EF679429 | EF679506 |
| C. sinense | CBS 143363* | MF473252 | MF473675 | MF474102 |
| C. sinuatum | CGMCC 3.18096* | KX938385 | KX938402 | KX938368 |
| C. sinuosum | CBS 121629* | EF679386 | EF679464 | EF679540 |
| C. sloanii | CBS 143364* | MF473253 | MF473676 | MF474103 |
| C. soldanellae | CBS 132186* | JN906982 | JN906994 | JN907001 |
| C. sphaerospermum# | CBS 193.54* | DQ780343 | EU570261 | EF101380 |
| C. spinulosum | CBS 119907* | EF679388 | EF679466 | EF679542 |
| C. subcinereum | CBS 140465* | LN834433 | LN834529 | LN834617 |
| C. subinflatum | CBS 121630* | EF679389 | EF679467 | EF679543 |
| C. subtilissimum# | CBS 113754* | EF679397 | EF679475 | EF679551 |
| C. subuliforme# | CBS 126500* | HM148196 | HM148441 | HM148686 |
| C. succulentum | CBS 140466* | LN834434 | LN834530 | LN834618 |
| C. tenellum# | CBS 121634* | EF679401 | EF679479 | EF679555 |
| C. tenuissimum# | CBS 125995* | HM148197 | HM148442 | HM148687 |
| C. tianshanense | CGMCC 3.18033* | KX938381 | KX938364 | |
| C. tuberosum | CBS 140693* | LN834417 | LN834513 | LN834601 |
| C. uredinicola | ATCC 46649 | AY251071 | HM148467 | HM148712 |
| C. uwebraunianum | CBS 143365* | MF473306 | MF473729 | MF474156 |
| C. variabile# | CBS 121635* | EF679402 | EF679480 | EF679556 |
| C. varians | CBS 126362* | HM148224 | HM148470 | HM148715 |
| C. velox | CBS 119417* | DQ780361 | JN906995 | EF101388 |
| C. verrucocladosporioides | CBS 126363* | HM148226 | HM148472 | HM148717 |
| C. verruculosum | CGMCC 3.18099* | KX938388 | KX938405 | KX938371 |
| C. versiforme | CBS 140491* | KT600417 | KT600515 | KT600613 |
| C. vicinum | CBS 143366* | MF473311 | MF473734 | MF474161 |
| C. vignae# | CBS 121.25 | HM148227 | HM148473 | HM148718 |
| C. welwitschiicola | CBS 142614* | KY646223 | KY646229 | KY646226 |
| C. westerdijkiae | CBS 113746* | HM148061 | HM148303 | HM148548 |
| C. wyomingense | CBS 143367* | MF473315 | MF473738 | MF474165 |
| C. xanthochromaticum | CBS 140691* | LN834415 | LN834511 | LN834599 |
| C. xylophilum | CBS 125997* | HM148230 | HM148476 | HM148721 |
| Toxicocladosporium banksiae | CBS 128215* | HQ599598 | LT821371 |
Fig 2 Phylogram generated from Maximum Likelihood analysis based on ITS, tef1 and act sequenced data. Bootstrap support values ≥75% and Bayesian posterior probabilities ≥0.95 are given near the nodes. The ex-type (ex-epitype) and voucher strains are in bold. The tree is rooted with Toxicocladosporium banksiae CBS 128215.



No Comments