16 Oct Rosellinia
Rosellinia De Not., G. bot. ital.1 (1): 334(1844)
Rosellinia (Xylariaceae) species are characterized mainly as saprobes, some endophytes and occasionally as pathogens. They have a worldwide distribution and common in both temperate and tropical regions (Petrini 1993, 2013; Hoopen and Krauss 2006). Plant pathogenic Roselleina species play a vital role in economically important crops, trees, and ornamental plants. Rosellinia desmazieresii and R. necatrix are mostly known from temperate regions, while R. bunodes is known only from the tropics causing root rot on fruit trees and vines (Agrios 2005; Hoopen and Krauss 2006). Among the root diseases cause by Rosellinia species, R. bunodes is responsible for black root rot, R. necatrix for white root rot and R. pepo for stellate root rot (Castro et al. 2013). Species of this genus can survive as microsclerotia in wood, roots, and soil and the infection spreads through feeder roots when they contact hyphae or microsclerotia (Ploetz et al. 2003).
Rosellinia was introduced to accommodate species that are characterized by uniascomatal and carbonaceous stromata that develop within a subiculum. There have been different contradictions placements of Rosellinia. Miller (1928) placed it in the family Xylariaceae and this was confirmed in morphology and phylogeny-based studies later (Hsieh et al. 2010; Daranagama et al. 2015). Daranagama et al. (2018) and Wendt et al. (2018) revealed that Rosellinia is closely related to Entoleuca and Nemania.
Classification – Sordariomycetes, Xylariomycetidae, Xylariales, Xylariaceae
Type species – Rosellinia aquila (Fr.) Ces. & De Not., G. bot. ital.1 (1): 334(1844)
Distribution – Worldwide
Disease symptoms – Root rots
Black root rot is characterized by the occurrence in patches that extend in a circular pattern. Rosellinia bundones the main causal agent of black root rot typically show black branching strands that are firmly attached to the roots and may form condensed irregular knots and chlorotic leaves may shed gradually (Sivanesan and Holiday 1972; Oliverira et al. 2008).
The symptoms of white root rot caused by R. necatrix in the upper parts of the plants (such as yellow foliage, shriveled fruits, no new growth) cannot be recognized in the early stages of root infection. Cottony, white mycelia cover feeder roots of a tree and decay sets in. Mycelia grow into the soil and upward in the tree forming small, pale patches under or in the bark of major roots, root crown and lower trunk which eventually decay. A purple canker in wood at the root crown of young trees can also be caused by the fungus. Diseased trees will defoliate and premature death may occur (Pérez-Jiménez 2006; Pasini et al. 2016).
Hosts – This genus has a wide range of hosts including Adoxaceae, Annonaceae, Apiaceae, Asteraceae, Betulaceae, Celastraceae, Convolvulaceae, Euphorbiaceae, Fabaceae, Fagaceae, Grossulariaceae, Juglandaceae, Lauraceae, Moraceae, Myrtaceae, Oleaceae, Pinaceae, Poaceae, Rosaceae, Rutaceae, Salicaceae, Sapindaceae, Scrophulariaceae, Tamaricaceae, Verbenaceae, Vitaceae and Zingiberaceae.
Morphological based identification and diversity
The genus is characterized by globose-subglobose, uni- to multiloculate, often collapsed ascomata, mostly detached from the stroma wall,; septate, hyaline paraphyses, asci that are 8-spored, unitunicate, cylindrical to clavate, long pedicellate, rounded at the apex, with J+ apical ring bluing in Melzer’s reagent, massive barrel-shaped with distinctive rings ascospores that are uniseriate, unicellular, elongated ellipsoidal-fusiform, light to dark brown, with germ slits, cellular appendages and/or maybe slimy sheaths or caps and a dematophora-like or geniculosporium-like asexual morphs (Daranagama et al. 2018).
Rosellinia is a large genus with 483 epithets in Mycobank, 517 in Index Fungorum and 311 in Global Biodiversity Information Facility (GBIF); there are currently accepted 158 species (Petrini 2013; Li and Guo 2015; Li et al. 2015, 2016; Su et al. 2016; Crous et al. 2017; Fournier et al. 2017; Tibpromma et al. 2017). Petrini (2013) found seven morphologically distinct groups with distinguishing morphological characters associated with the shape, size, and orientations of stroma, ostiole, ascospores and germ slit, which can be used for species delimitation.
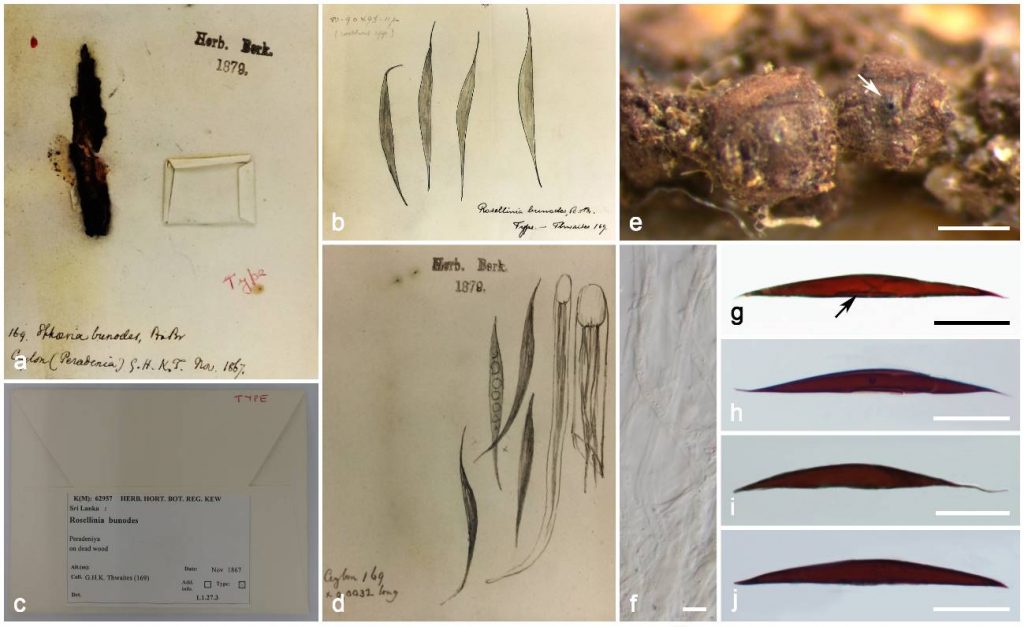
Fig. Rosellinia bunodes (holotype, K(M) 62957, SRI LANKA, Peradeniya, on dead wood, November 1867, G.H.K. Thwaites) a,e. Stromata (e ostiole white arrow). b–d. Herbarium details. f. Paraphyses. g–j. Ascospores (g germ slit black arrow). Scale bars: e = 500 µm, g–j = 20 µm, f = 5 µm.
Molecular based identification and diversity
Protein coding gene sequences are available for nine species of Rosellinia, mostly with only ITS and LSU sequence data. However, with the limited data, Daranagama et al. (2018) provided an updated backbone tree for genera in Xylariaceae, and Rossellinia clustered with Nemania and Entoleuca. Several phylogenetic studies have focused on pathogenic species such as R. bunodes and R. pepo. Castro et al. (2013) investigated R. bunodes and R. pepo isolated from Coffea arabica (Rubiaceae), Hevea brasiliensis (Euphorbiaceae), Macadamia integrifolia (Proteaceae), Psidium guajava (Myrtaceae) and Theobroma cacao (Malvaceae) using ITS based phylogenetic analyses from Colombia. Another ITS-based phylogenetic study identified R. necatrix, the pathogen responsible for white root disease on Aronia melanocarpa (Rosaceae) in Korea (Choi et al. 2017).
This study reconstructs the phylogeny of Rosellinia based on analyses of combined ITS, LSU and RPB2 sequence data (Table 7, Fig. 13). The phylogenetic tree is updated with recently introduced Rosellinia species and corresponds to previous studies (Li et al. 2015, 2016; Su et al. 2016; Crous et al. 2017; Fournier et al. 2017; Tibpromma et al. 2017).
Recommended genetic markers (genus level) – LSU, ITS
Recommended genetic markers (species level) – ITS
Based on several studies and the availability of sequence data, ITS based phylogenetic studies are sufficient to identify Rosellinia to species level. There are few other studies carried out using LSU, ITS, and RPB2 sequences. With a lack of sequence data for most species, there are some contradictions for the species and generic delimitation.
The accepted number of species: 158
References: Petrini 2013; Li and Guo 2015; (morphology), Castro et al. 2013; Li et al. 2015, 2016; Crous et al. 2017; Fournier et al. 2017; Tibpromma et al. 2017, Daranagama et al. 2018 (morphology, phylogeny), Shimizu et al. 2012; dos Santos et al. 2017; Arjona-Girona and López-Herrera 2018; Kleina et al. 2018 (pathogenicity).

No Comments